OsACOS12, an orthologue of Arabidopsis acyl-CoA synthetase5, plays an important role in pollen exine formation and anther development in rice
- PMID: 27871243
- PMCID: PMC5117612
- DOI: 10.1186/s12870-016-0943-9
OsACOS12, an orthologue of Arabidopsis acyl-CoA synthetase5, plays an important role in pollen exine formation and anther development in rice
Abstract
Background: Sporopollenin is a major component of the pollen exine pattern. In Arabidopsis, acyl-CoA synthetase5 (ACOS5) is involved in sporopollenin precursor biosynthesis. In this study, we identified its orthologue, OsACOS12, in rice (Oryza sativa) and compared the functional conservation of ACOS in rice to Arabidopsis.
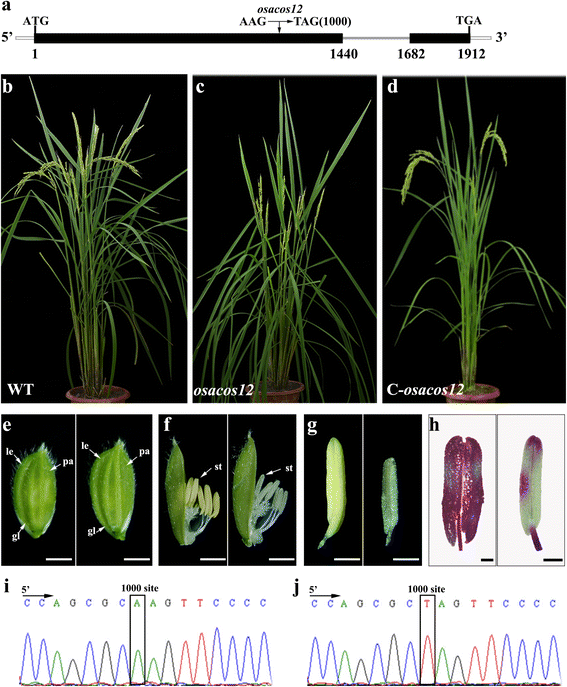
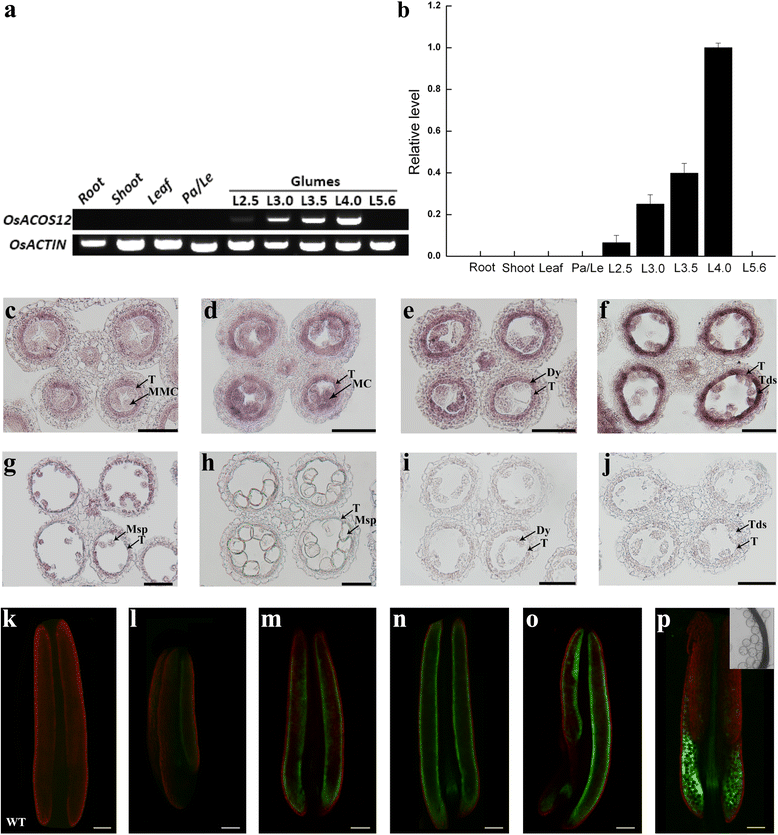
Results: Sequence analysis showed that OsACOS12 shares 63.9 % amino acid sequence identity with ACOS5. The osacos12 mutation caused by a pre-mature stop codon in LOC_Os04g24530 exhibits defective sexine resulting in a male sterile phenotype in rice. In situ hybridization shows that OsACOS12 is expressed in tapetal cells and microspores at the transcript level. The localization of OsACOS12-GFP demonstrated that OsACOS12 protein is accumulated in tapetal cells and anther locules. OsACOS12 driven by the ACOS5 promoter could partially restore the male fertility of the acos5 mutant in Arabidopsis.
Conclusions: OsACOS12 is an orthologue of ACOS5 that is essential for sporopollenin synthesis in rice. ACOS5 and OsACOS12 are conserved for pollen wall formation in monocot and dicot species.
Keywords: Anther cuticle; Male sterility; Oryza sativa; OsACOS12; Pollen exine.
Figures




Similar articles
-
Acyl-CoA synthetases from Physcomitrella, rice and Arabidopsis: different substrate preferences but common regulation by MS188 in sporopollenin synthesis.Planta. 2019 Aug;250(2):535-548. doi: 10.1007/s00425-019-03189-0. Epub 2019 May 21. Planta. 2019. PMID: 31111205
-
Rice fatty acyl-CoA synthetase OsACOS12 is required for tapetum programmed cell death and male fertility.Planta. 2017 Jul;246(1):105-122. doi: 10.1007/s00425-017-2691-y. Epub 2017 Apr 5. Planta. 2017. PMID: 28382520
-
A novel fatty Acyl-CoA Synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis.Plant Cell. 2009 Feb;21(2):507-25. doi: 10.1105/tpc.108.062513. Epub 2009 Feb 13. Plant Cell. 2009. PMID: 19218397 Free PMC article.
-
Rice anther tapetum: a vital reproductive cell layer for sporopollenin biosynthesis and pollen exine patterning.Plant Biol (Stuttg). 2023 Mar;25(2):233-245. doi: 10.1111/plb.13485. Epub 2022 Dec 16. Plant Biol (Stuttg). 2023. PMID: 36350096 Review.
-
The biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack.Phytochemistry. 2015 May;113:170-82. doi: 10.1016/j.phytochem.2014.05.002. Epub 2014 Jun 3. Phytochemistry. 2015. PMID: 24906292 Review.
Cited by
-
Biological Roles of Lipids in Rice.Int J Mol Sci. 2024 Aug 21;25(16):9046. doi: 10.3390/ijms25169046. Int J Mol Sci. 2024. PMID: 39201734 Free PMC article. Review.
-
CRISPR/Cas9-mediated disruption of CjACOS5 confers no-pollen formation on sugi trees (Cryptomeria japonica D. Don).Sci Rep. 2023 Jul 21;13(1):11779. doi: 10.1038/s41598-023-38339-8. Sci Rep. 2023. PMID: 37479866 Free PMC article.
-
The Toughest Material in the Plant Kingdom: An Update on Sporopollenin.Front Plant Sci. 2021 Sep 3;12:703864. doi: 10.3389/fpls.2021.703864. eCollection 2021. Front Plant Sci. 2021. PMID: 34539697 Free PMC article. Review.
-
Transcriptomic and Proteomic Analyses of Celery Cytoplasmic Male Sterile Line and Its Maintainer Line.Int J Mol Sci. 2023 Feb 20;24(4):4194. doi: 10.3390/ijms24044194. Int J Mol Sci. 2023. PMID: 36835607 Free PMC article.
-
Acyl-CoA synthetases from Physcomitrella, rice and Arabidopsis: different substrate preferences but common regulation by MS188 in sporopollenin synthesis.Planta. 2019 Aug;250(2):535-548. doi: 10.1007/s00425-019-03189-0. Epub 2019 May 21. Planta. 2019. PMID: 31111205
References
-
- Scott RJ. Pollen exine: The sporopollenin enigma and the physics of pattern. In: Scott RJ, Stead AD, editors. Molecular and Cellular Aspects of Plant Reproduction. Cambridge, UK: Cambrige University Press; 1994. pp. 49–81.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

