Identification of genes for engineering the male germline of Aedes aegypti and Ceratitis capitata
- PMID: 27871244
- PMCID: PMC5117610
- DOI: 10.1186/s12864-016-3280-3
Identification of genes for engineering the male germline of Aedes aegypti and Ceratitis capitata
Abstract
Background: Synthetic biology approaches are promising new strategies for control of pest insects that transmit disease and cause agricultural damage. These strategies require characterised modular components that can direct appropriate expression of effector sequences, with components conserved across species being particularly useful. The goal of this study was to identify genes from which new potential components could be derived for manipulation of the male germline in two major pest species, the mosquito Aedes aegypti and the tephritid fruit fly Ceratitis capitata.
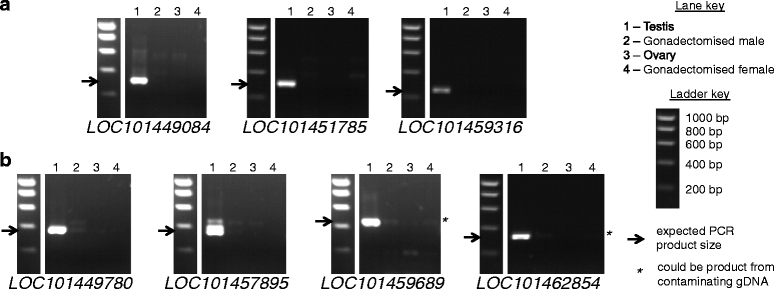
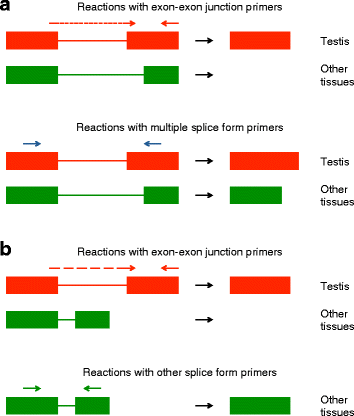
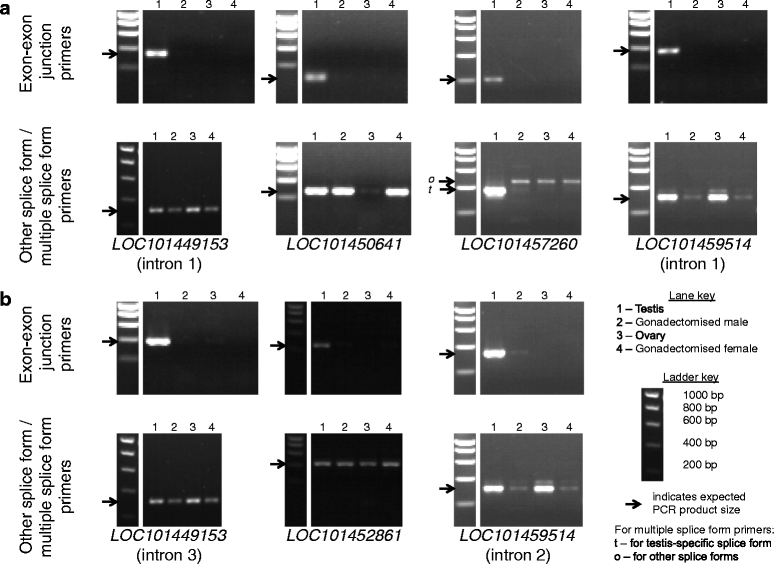
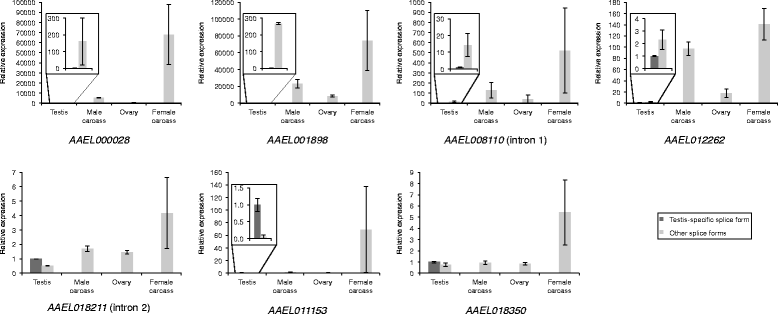
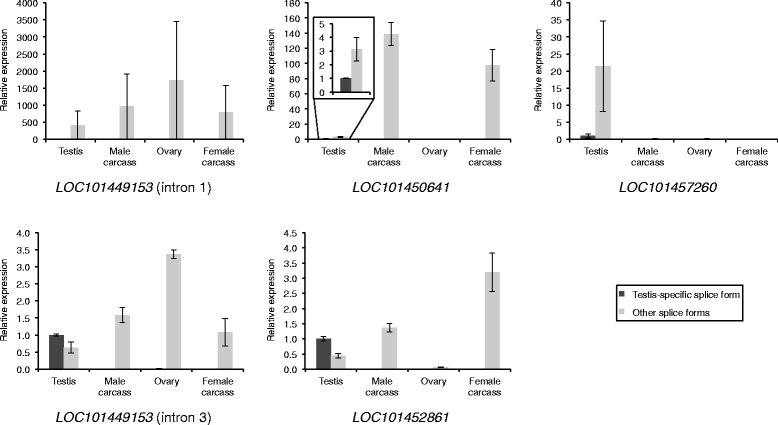
Results: Using RNA-seq data from staged testis samples, we identified several candidate genes with testis-specific expression and suitable expression timing for use of their regulatory regions in synthetic control constructs. We also developed a novel computational pipeline to identify candidate genes with testis-specific splicing from this data; use of alternative splicing is another method for restricting expression in synthetic systems. Some of the genes identified display testis-specific expression or splicing that is conserved across species; these are particularly promising candidates for construct development.
Conclusions: In this study we have identified a set of genes with testis-specific expression or splicing. In addition to their interest from a basic biology perspective, these findings provide a basis from which to develop synthetic systems to control important pest insects via manipulation of the male germline.
Keywords: Aedes aegypti; Ceratitis capitata; Male germline; Pest insect; RNA-seq; Synthetic biology.
Figures






Similar articles
-
Transcriptional profiles of mating-responsive genes from testes and male accessory glands of the Mediterranean fruit fly, Ceratitis capitata.PLoS One. 2012;7(10):e46812. doi: 10.1371/journal.pone.0046812. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071645 Free PMC article.
-
Sniffing out chemosensory genes from the Mediterranean fruit fly, Ceratitis capitata.PLoS One. 2014 Jan 8;9(1):e85523. doi: 10.1371/journal.pone.0085523. eCollection 2014. PLoS One. 2014. PMID: 24416419 Free PMC article.
-
Gene discovery in an invasive tephritid model pest species, the Mediterranean fruit fly, Ceratitis capitata.BMC Genomics. 2008 May 23;9:243. doi: 10.1186/1471-2164-9-243. BMC Genomics. 2008. PMID: 18500975 Free PMC article.
-
How functional genomics will impact fruit fly pest control: the example of the Mediterranean fruit fly, Ceratitis capitata.BMC Genet. 2014;15 Suppl 2(Suppl 2):S11. doi: 10.1186/1471-2156-15-S2-S11. Epub 2014 Dec 1. BMC Genet. 2014. PMID: 25471105 Free PMC article. Review.
-
Polytene chromosomes as tools in the genetic analysis of the Mediterranean fruit fly, Ceratitis capitata.Genetica. 2002 Sep;116(1):59-71. doi: 10.1023/a:1020959608886. Genetica. 2002. PMID: 12484526 Review. No abstract available.
Cited by
-
The Challenges in Developing Efficient and Robust Synthetic Homing Endonuclease Gene Drives.Front Bioeng Biotechnol. 2022 Mar 28;10:856981. doi: 10.3389/fbioe.2022.856981. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35419354 Free PMC article. Review.
-
Differential gene expression underpinning the production of distinct sperm morphs in the wax moth Galleria mellonella.Open Biol. 2024 Jul;14(7):240002. doi: 10.1098/rsob.240002. Epub 2024 Jul 31. Open Biol. 2024. PMID: 39079672 Free PMC article.
-
Aedes Anphevirus: an Insect-Specific Virus Distributed Worldwide in Aedes aegypti Mosquitoes That Has Complex Interplays with Wolbachia and Dengue Virus Infection in Cells.J Virol. 2018 Aug 16;92(17):e00224-18. doi: 10.1128/JVI.00224-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29950416 Free PMC article.
-
Proteins, Transcripts, and Genetic Architecture of Seminal Fluid and Sperm in the Mosquito Aedes aegypti.Mol Cell Proteomics. 2019 Mar 15;18(Suppl 1):S6-S22. doi: 10.1074/mcp.RA118.001067. Epub 2018 Dec 14. Mol Cell Proteomics. 2019. PMID: 30552291 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- BB/H016473/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001892/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L004445/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J012696/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

