Epidemiology and RAPD-PCR typing of thermophilic campylobacters from children under five years and chickens in Morogoro Municipality, Tanzania
- PMID: 27871251
- PMCID: PMC5117500
- DOI: 10.1186/s12879-016-2031-z
Epidemiology and RAPD-PCR typing of thermophilic campylobacters from children under five years and chickens in Morogoro Municipality, Tanzania
Abstract
Background: Campylobacter species are gram negative and flagellated bacteria under the genus Campylobacter, family Campylobacteriaceae. These pathogens cause zoonotic infections among human and animal populations. This study was undertaken between December 2006 and May 2007 to determine prevalence, risk factors and genetic diversity of thermophilic Campylobacter isolates from children less than 5 years and chickens in Morogoro Municipality, Tanzania.
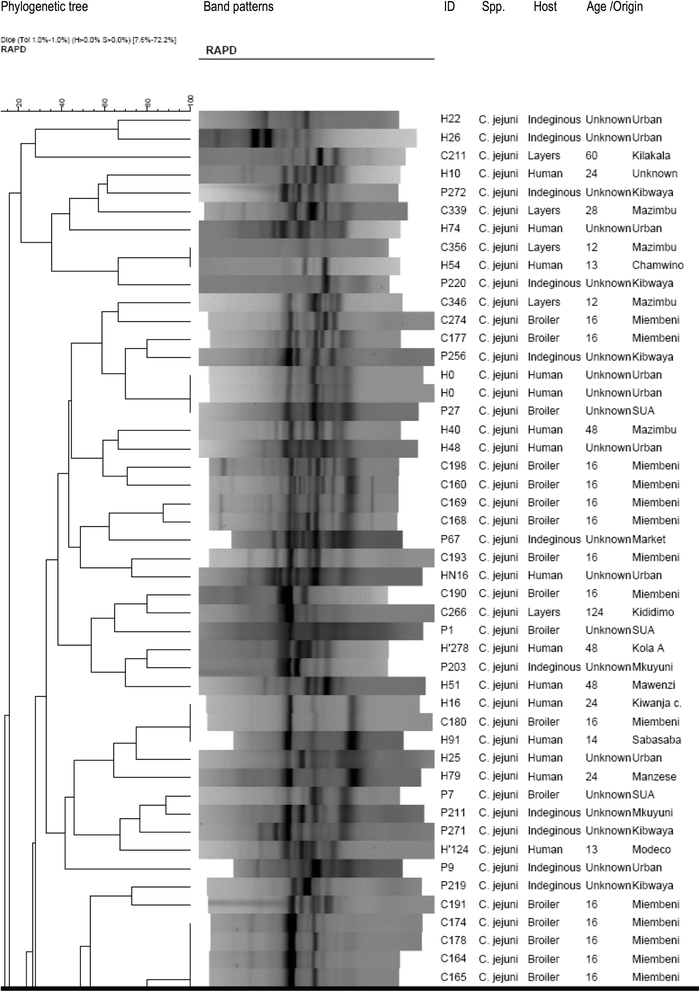
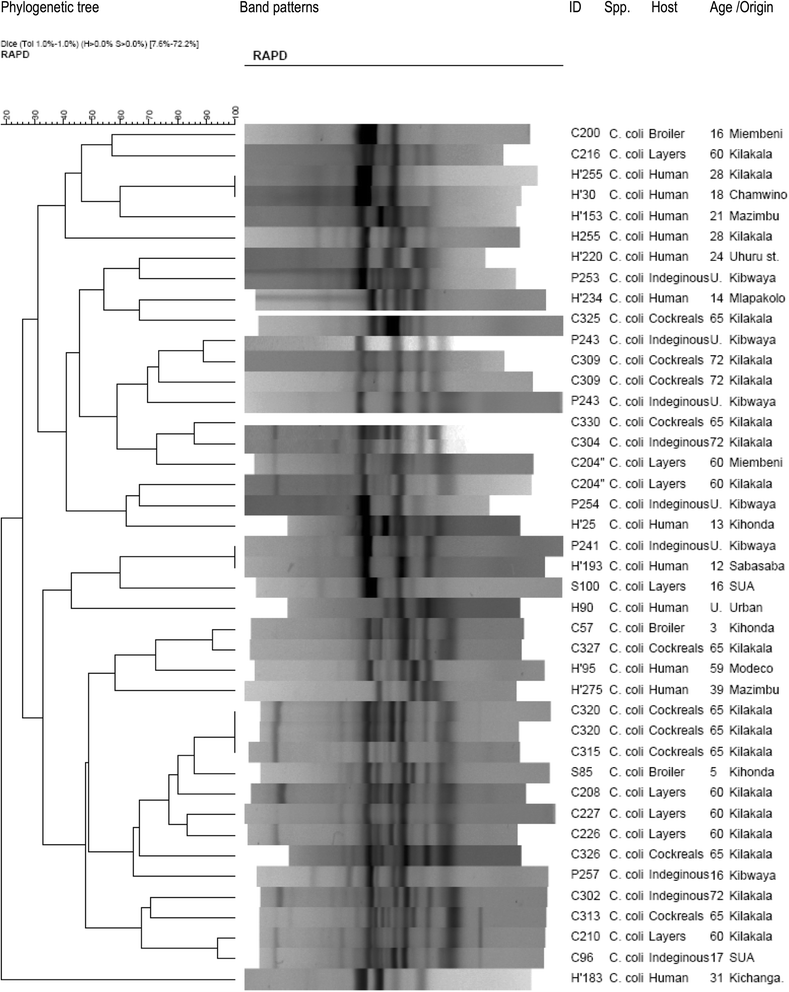
Methods: The Skirrow's protocol was used for isolation and identification of Campylobacter from 268 human stool specimens and 419 chicken cloacal swabs. Patient biodata and risk factors associated with human infection were also collected. Genetic diversity of Campylobacter isolates was determined by a RAPD-PCR technique using OPA 11 primer (5'-CAA TCG CCG T-3'). Phylogenetic analysis and band pattern comparison were done by Bionumerics software and visual inspection.
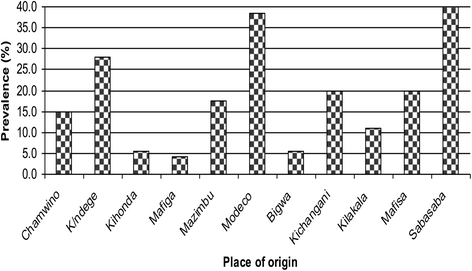
Results: Stool samples from 268 children and 419 cloacal swabs from chickens were analyzed. Prevalence of thermophilic Campylobacters in children was 19% with higher isolation frequency (p = 0.046) in males (23.5%) than females (13.8%). Campylobacter jejuni (78.4%) was more isolated (p = 0.000) than C. coli (19.6%) and 2% were unidentified isolates. In chickens, the prevalence was 42.5% with higher isolation rate (p = 0.000) of C. jejuni (87%) than C. coli (13%). Campylobacters were more frequently recovered (p = 0.000) from indigenous/ local chickens (75.0%) followed by cockerels (52.2%), broilers (50.0%) and lowest in layers (22.7%). Keeping chickens without other domestic animals concurrently (p = 0.000), chicken types (p = 0.000) and flock size (p = 0.007) were risk factors for infection in chickens. One hundred and fifty two (152) thermophillic Campylobacter isolates were genotyped by RAPD-PCR of which 114 were C. jejuni (74 from chickens and 40 humans) and 38 C. coli (28 from chickens and 10 humans). Comparison of Campylobacter isolates from children and chickens revealed high diversity with only 6.1% of C. jejuni and 5.3% of C. coli being 100% genetically similar.
Conclusions: This study has recorded high prevalence of thermophilic Campylobacter in children less than 5 years and chickens in Morogoro municipality. The observed genetic similarity among few C. jejuni and C. coli isolates from children and chicken suggests existence of cross transmission of these pathogens between children under 5 years and chickens.
Keywords: C. coli; Campylobacter jejuni; Chicken; Children; Epidemiology; Genetic diversity; Morogoro; RAPD PCR; Tanzania.
Figures
Similar articles
-
Prevalence of thermophilic campylobacter infections in humans, chickens and crows in Morogoro, Tanzania.J Vet Med B Infect Dis Vet Public Health. 2006 Apr;53(3):116-21. doi: 10.1111/j.1439-0450.2006.00926.x. J Vet Med B Infect Dis Vet Public Health. 2006. PMID: 16629722
-
Comparison of Cape Town and Skirrow's Campylobacter isolation protocols in humans and broilers in Morogoro, Tanzania.Trop Anim Health Prod. 2011 Jun;43(5):1007-13. doi: 10.1007/s11250-011-9799-z. Epub 2011 Feb 26. Trop Anim Health Prod. 2011. PMID: 21359592
-
Prevalence and antibiotic susceptibility of thermophilic Campylobacter isolates from free range domestic duck (Cairina moschata) in Morogoro municipality, Tanzania.Trop Anim Health Prod. 2010 Feb;42(2):165-72. doi: 10.1007/s11250-009-9401-0. Epub 2009 Jun 28. Trop Anim Health Prod. 2010. PMID: 19562499
-
Poultry as a host for the zoonotic pathogen Campylobacter jejuni.Vector Borne Zoonotic Dis. 2012 Feb;12(2):89-98. doi: 10.1089/vbz.2011.0676. Epub 2011 Dec 1. Vector Borne Zoonotic Dis. 2012. PMID: 22133236 Review.
-
A systematic review of source attribution of human campylobacteriosis using multilocus sequence typing.Euro Surveill. 2019 Oct;24(43):1800696. doi: 10.2807/1560-7917.ES.2019.24.43.1800696. Euro Surveill. 2019. PMID: 31662159 Free PMC article.
Cited by
-
A One Health Approach to Child Stunting: Evidence and Research Agenda.Am J Trop Med Hyg. 2021 Mar 8;104(5):1620-1624. doi: 10.4269/ajtmh.20-1129. Am J Trop Med Hyg. 2021. PMID: 33684062 Free PMC article. Review.
-
Significant contribution of the CmeABC Efflux pump in high-level resistance to ciprofloxacin and tetracycline in Campylobacter jejuni and Campylobacter coli clinical isolates.Ann Clin Microbiol Antimicrob. 2021 May 20;20(1):36. doi: 10.1186/s12941-021-00439-6. Ann Clin Microbiol Antimicrob. 2021. PMID: 34016127 Free PMC article.
-
Population Structure and Antimicrobial Resistance in Campylobacter jejuni and C. coli Isolated from Humans with Diarrhea and from Poultry, East Africa.Emerg Infect Dis. 2024 Oct;30(10):2079-2089. doi: 10.3201/eid3010.231399. Emerg Infect Dis. 2024. PMID: 39320160 Free PMC article.
-
Genomic Insights into the Increased Occurrence of Campylobacteriosis Caused by Antimicrobial-Resistant Campylobacter coli.mBio. 2022 Dec 20;13(6):e0283522. doi: 10.1128/mbio.02835-22. Epub 2022 Dec 6. mBio. 2022. PMID: 36472434 Free PMC article.
-
Molecular determination of genetic diversity among Campylobacter jejuni and Campylobacter coli isolated from milk, water, and meat samples using enterobacterial repetitive intergenic consensus PCR (ERIC-PCR).Infect Ecol Epidemiol. 2020 Oct 6;10(1):1830701. doi: 10.1080/20008686.2020.1830701. Infect Ecol Epidemiol. 2020. PMID: 33133420 Free PMC article.
References
-
- Nachamkin I, Blaser MJ. Campylobacteriosis. 2. Washington: American Society for Microbiology; 2000.
-
- Mdegela RH, Nonga HE, Ngowi HA, Kazwala RR. Prevalence of thermophilic campylobacter infection in humans, chickens and Crows in Morogoro, Tanzania. J Vet Med. 2006;B 53:116–21. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

