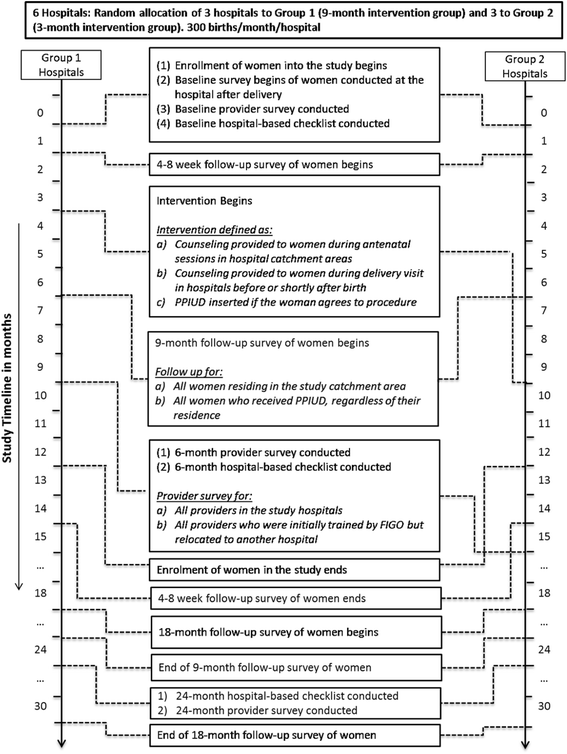
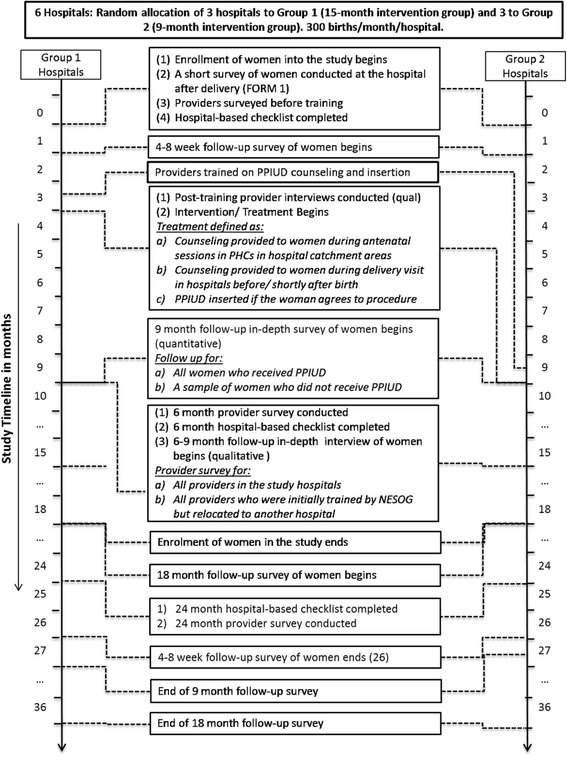
Institutionalizing postpartum intrauterine device (IUD) services in Sri Lanka, Tanzania, and Nepal: study protocol for a cluster-randomized stepped-wedge trial
- PMID: 27871269
- PMCID: PMC5117577
- DOI: 10.1186/s12884-016-1160-0
Institutionalizing postpartum intrauterine device (IUD) services in Sri Lanka, Tanzania, and Nepal: study protocol for a cluster-randomized stepped-wedge trial
Abstract
Background: During the year following the birth of a child, 40% of women are estimated to have an unmet need for contraception. The copper IUD provides safe, effective, convenient, and long-term contraceptive protection that does not interfere with breastfeeding during the postpartum period. Postpartum IUD (PPIUD) insertion should be performed by a trained provider in the early postpartum period to reduce expulsion rates and complications, but these services are not widely available. The International Federation of Obstetricians and Gynecologists (FIGO) will implement an intervention that aims to institutionalize PPIUD training as a regular part of the OB/GYN training program and to integrate it as part of the standard practice at the time of delivery in intervention hospitals.
Methods: This trial uses a cluster-randomized stepped wedge design to assess the causal effect of the FIGO intervention on the uptake and continued use of PPIUD and of the effect on subsequent pregnancy and birth. This trial also seeks to measure institutionalization of PPIUD services in study hospitals and diffusion of these services to other providers and health facilities. This study will also include a nested mixed-methods performance evaluation to describe intervention implementation.
Discussion: This study will provide critical evidence on the causal effects of hospital-based PPIUD provision on contraceptive choices and reproductive health outcomes, as well as on the feasibility, acceptability and longer run institutional impacts in three low- and middle-income countries.
Trial registration: Trial registered on March 11, 2016 with ClinicalTrials.gov, NCT02718222 .
Keywords: IUD; Impact evaluation; Postpartum contraception.
Figures
Similar articles
-
Integrating postpartum contraceptive counseling and IUD insertion services into maternity care in Nepal: results from stepped-wedge randomized controlled trial.Reprod Health. 2019 May 29;16(1):69. doi: 10.1186/s12978-019-0738-1. Reprod Health. 2019. PMID: 31142344 Free PMC article. Clinical Trial.
-
The effect of a postpartum IUD intervention on counseling and choice: Evidence from a cluster-randomized stepped-wedge trial in Sri Lanka.Trials. 2019 Jul 8;20(1):407. doi: 10.1186/s13063-019-3473-6. Trials. 2019. PMID: 31287021 Free PMC article.
-
FIGO postpartum intrauterine device initiative: Complication rates across six countries.Int J Gynaecol Obstet. 2018 Sep;143 Suppl 1:20-27. doi: 10.1002/ijgo.12600. Int J Gynaecol Obstet. 2018. PMID: 30225873
-
Immediate postpartum provision of long-acting reversible contraception.Curr Opin Obstet Gynecol. 2015 Dec;27(6):460-4. doi: 10.1097/GCO.0000000000000224. Curr Opin Obstet Gynecol. 2015. PMID: 26536209 Review.
-
Intrauterine contraception.Curr Opin Obstet Gynecol. 1992 Aug;4(4):527-30. Curr Opin Obstet Gynecol. 1992. PMID: 1324024 Review.
Cited by
-
Evaluating the Implementation of an Intervention to Improve Postpartum Contraception in Tanzania: A Qualitative Study of Provider and Client Perspectives.Glob Health Sci Pract. 2020 Jun 30;8(2):270-289. doi: 10.9745/GHSP-D-19-00365. Print 2020 Jun 30. Glob Health Sci Pract. 2020. PMID: 32606094 Free PMC article.
-
Integrating postpartum IUD counselling and insertion into routine maternity care in Nepal: Assessing trends over time.PLOS Glob Public Health. 2023 Mar 22;3(3):e0001665. doi: 10.1371/journal.pgph.0001665. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 36963067 Free PMC article.
-
A maximum likelihood approach to power calculations for stepped wedge designs of binary outcomes.Biostatistics. 2020 Jan 1;21(1):102-121. doi: 10.1093/biostatistics/kxy031. Biostatistics. 2020. PMID: 30084949 Free PMC article.
-
A one-year cohort study of complications, continuation, and failure rates of postpartum TCu380A in Tanzania.Reprod Health. 2020 Oct 6;17(1):150. doi: 10.1186/s12978-020-00999-4. Reprod Health. 2020. PMID: 33023611 Free PMC article.
-
Immediate postpartum use of long-acting reversible contraceptives in low- and middle-income countries.Matern Health Neonatol Perinatol. 2017 Dec 22;3:24. doi: 10.1186/s40748-017-0063-z. eCollection 2017. Matern Health Neonatol Perinatol. 2017. PMID: 29299334 Free PMC article. Review.
References
-
- Winfrey W, Kshitiz R. Use of Family Planning in Postpartum Period. 2014, 36.
-
- World Health Organization (WHO). Medical Eligibility Criteria for Contraceptive use: Fourth Edition, 2009. 2010. - PubMed
-
- Grimes DA, Lopez LM, Schulz KF. Van Vilet HAAM. Stanwood NL: Immediate post-partum insertion of intrauterine devices. Cochrane Database of Systematic Reviews; 2010. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous