An innovative and collaborative partnership between patients with rare disease and industry-supported registries: the Global aHUS Registry
- PMID: 27871301
- PMCID: PMC5117495
- DOI: 10.1186/s13023-016-0537-5
An innovative and collaborative partnership between patients with rare disease and industry-supported registries: the Global aHUS Registry
Abstract
Background: Patients are becoming increasingly involved in research which can promote innovation through novel ideas, support patient-centred actions, and facilitate drug development. For rare diseases, registries that collect data from patients can increase knowledge of the disease's natural history, evaluate clinical therapies, monitor drug safety, and measure quality of care. The active participation of patients is expected to optimise rare-disease management and improve patient outcomes. However, few reports address the type and frequency of interactions involving patients, and what research input patient groups have. Here, we describe a collaboration between an international group of patient organisations advocating for patients with atypical haemolytic uraemic syndrome (aHUS), the aHUS Alliance, and an international aHUS patient registry (ClinicalTrials.gov NCT01522183).
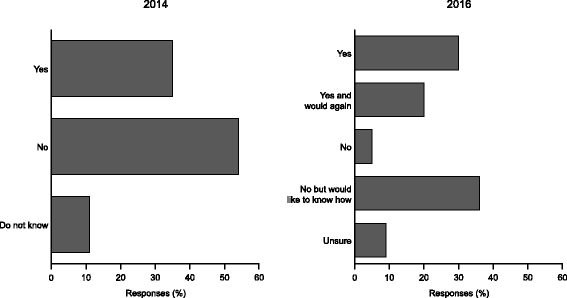
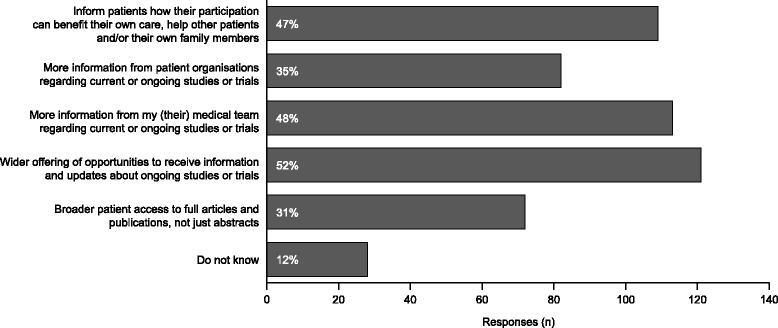
Results: The aHUS Registry Scientific Advisory Board (SAB) invited the aHUS Alliance to submit research ideas important to patients with aHUS. This resulted in 24 research suggestions from patients and patient organisations being presented to the SAB. The proposals were classified under seven categories, the most popular of which were understanding factors that cause disease manifestations and learning more about the clinical and psychological/social impact of living with the disease. Subsequently, aHUS Alliance members voted for up to five research priorities. The top priority was: "What are the outcomes of a transplant without eculizumab and what non-kidney damage is likely in patients with aHUS?". This led directly to the initiation of an ongoing analysis of the data collected in the Registry on patients with kidney transplants.
Conclusion: This collaboration resulted in several topics proposed by the aHUS Alliance being selected as priority activities for the aHUS Registry, with one new analysis already underway. A clear pathway was established for engagement between a patient advocacy group and an international research network. This should ensure the development of a long-term partnership which clearly benefits both groups.
Keywords: Patient advocacy; Patient engagement; Registry; aHUS.
Figures


Similar articles
-
The global aHUS registry: methodology and initial patient characteristics.BMC Nephrol. 2015 Dec 10;16:207. doi: 10.1186/s12882-015-0195-1. BMC Nephrol. 2015. PMID: 26654630 Free PMC article.
-
The NCI All Ireland Cancer Conference.Oncologist. 1999;4(4):275-277. Oncologist. 1999. PMID: 10545862
-
Atypical haemolytic uraemic syndrome treated with the complement inhibitor eculizumab: the experience of the Australian compassionate access cohort.Intern Med J. 2015 Oct;45(10):1054-65. doi: 10.1111/imj.12864. Intern Med J. 2015. PMID: 26247170
-
Suspected atypical haemolytic uraemic syndrome in two post-partum patients with foetal-death in utero responding to eculizumab.Nephrology (Carlton). 2017 Feb;22 Suppl 1:18-22. doi: 10.1111/nep.12935. Nephrology (Carlton). 2017. PMID: 28176472 Review.
-
Long-term remission with eculizumab in atypical haemolytic uraemic syndrome.Nephrology (Carlton). 2017 Feb;22 Suppl 1:7-10. doi: 10.1111/nep.12932. Nephrology (Carlton). 2017. PMID: 28176479 Review.
Cited by
-
Methods to Generate Innovative Research Ideas and Improve Patient and Public Involvement in Modern Epidemiological Research: Review, Patient Viewpoint, and Guidelines for Implementation of a Digital Cohort Study.J Med Internet Res. 2021 Dec 23;23(12):e25743. doi: 10.2196/25743. J Med Internet Res. 2021. PMID: 34941554 Free PMC article. Review.
-
Developing a community-led rare disease ELSI research agenda.Orphanet J Rare Dis. 2024 Jan 22;19(1):23. doi: 10.1186/s13023-023-02986-x. Orphanet J Rare Dis. 2024. PMID: 38254122 Free PMC article. Review.
-
Barriers to diverse clinical trial participation in Duchenne muscular dystrophy: Engaging Hispanic/Latina caregivers and health professionals.Orphanet J Rare Dis. 2024 May 21;19(1):207. doi: 10.1186/s13023-024-03209-7. Orphanet J Rare Dis. 2024. PMID: 38773664 Free PMC article.
-
Needs of people with rare diseases that can be supported by electronic resources: a scoping review.BMJ Open. 2022 Sep 1;12(9):e060394. doi: 10.1136/bmjopen-2021-060394. BMJ Open. 2022. PMID: 36581982 Free PMC article.
-
Demographics and baseline disease characteristics of UK patients within the global aHUS registry.BMC Nephrol. 2025 Aug 5;26(1):434. doi: 10.1186/s12882-025-04321-x. BMC Nephrol. 2025. PMID: 40764536 Free PMC article.
References
-
- Parsons S, Starling B, Mullan-Jensen C, Tham SG, Warner K, Wever K. What do pharmaceutical industry professionals in Europe believe about involving patients and the public in research and development of medicines? A qualitative interview study. BMJ Open. 2016;6(1):e008928. doi: 10.1136/bmjopen-2015-008928. - DOI - PMC - PubMed
-
- Workman TA. Engaging Patients in Information Sharing and Data Collection: The Role of Patient-Powered Registries and Research Networks. Rockville (MD): AHRQ Methods for Effective Health Care; 2013. - PubMed
-
- Gliklich RE, Dreyer NA, Leavy MA. (eds). Registries for Evaluating Patient Outcomes: A User’s Guide. Third edition. (Prepared by the Outcome DEcIDE Center [Outcome Sciences, Inc., a Quintiles company] under Contract No. 290 2005 00351 TO7). AHRQ Publication No. 13(14)-EHC111. Rockville, MD: Agency for Healthcare Research and Quality. 2014. http://www.effectivehealthcare.ahrq.gov/registries-guide-3.cfm. Accessed Nov 2016.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

