Akirin2 is essential for the formation of the cerebral cortex
- PMID: 27871306
- PMCID: PMC5117564
- DOI: 10.1186/s13064-016-0076-8
Akirin2 is essential for the formation of the cerebral cortex
Abstract
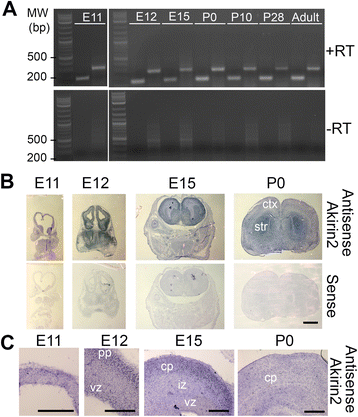
Background: The proper spatial and temporal regulation of dorsal telencephalic progenitor behavior is a prerequisite for the formation of the highly-organized, six-layered cerebral cortex. Premature differentiation of cells, disruption of cell cycle timing, excessive apoptosis, and/or incorrect neuronal migration signals can have devastating effects, resulting in a number of neurodevelopmental disorders involving microcephaly and/or lissencephaly. Though genes encoding many key players in cortical development have been identified, our understanding remains incomplete. We show that the gene encoding Akirin2, a small nuclear protein, is expressed in the embryonic telencephalon. Converging evidence indicates that Akirin2 acts as a bridge between transcription factors (including Twist and NF-κB proteins) and the BAF (SWI/SNF) chromatin remodeling machinery to regulate patterns of gene expression. Constitutive knockout of Akirin2 is early embryonic lethal in mice, while restricted loss in B cells led to disrupted proliferation and cell survival.
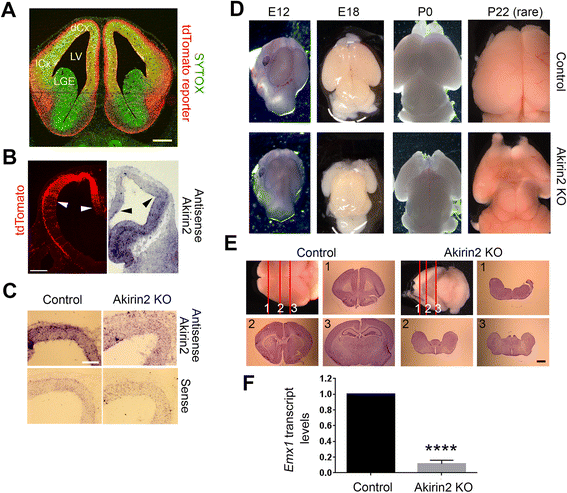
Methods: We generated cortex-restricted Akirin2 knockouts by crossing mice harboring a floxed Akirin2 allele with the Emx1-Cre transgenic line and assessed the resulting embryos using in situ hybridization, EdU labeling, and immunohistochemistry.
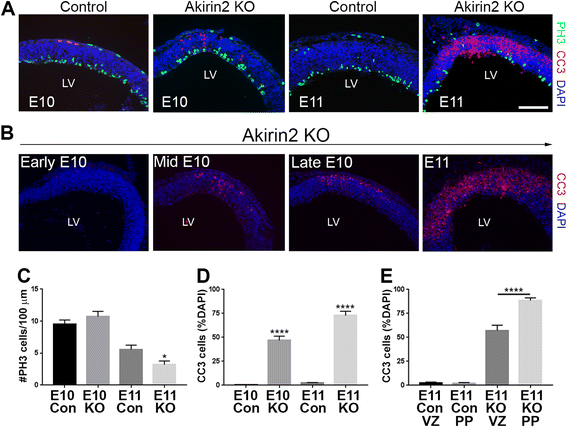
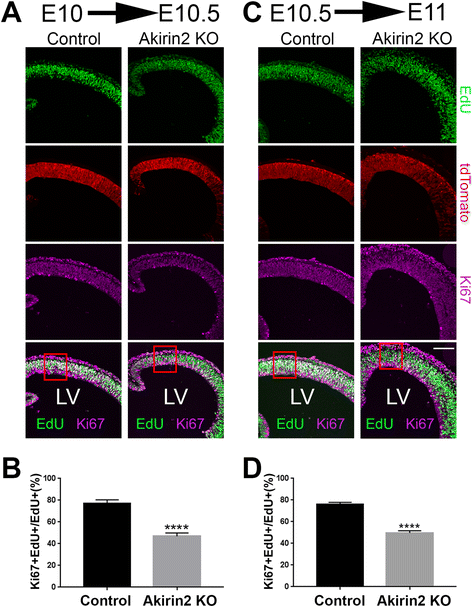
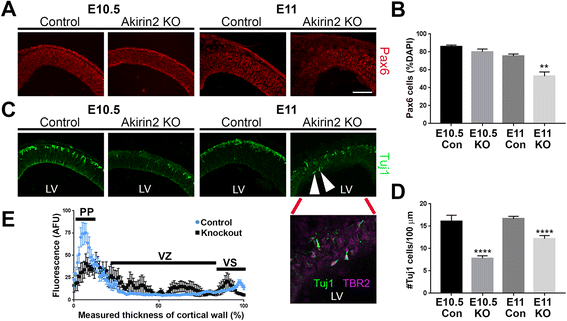
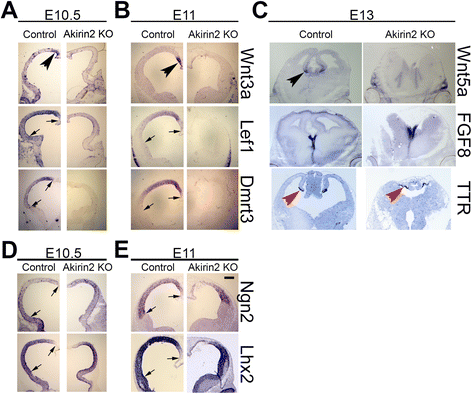
Results: The vast majority of Akirin2 mutants do not survive past birth, and exhibit extreme microcephaly, with little dorsal telencephalic tissue and no recognizable cortex. This is primarily due to massive cell death of early cortical progenitors, which begins at embryonic day (E)10, shortly after Emx1-Cre is active. Immunostaining and cell cycle analysis using EdU labeling indicate that Akirin2-null progenitors fail to proliferate normally, produce fewer neurons, and undergo extensive apoptosis. All of the neurons that are generated in Akirin2 mutants also undergo apoptosis by E12. In situ hybridization for Wnt3a and Wnt-responsive genes suggest defective formation and/or function of the cortical hem in Akirin2 null mice. Furthermore, the apical ventricular surface becomes disrupted, and Sox2-positive progenitors are found to "spill" into the lateral ventricle.
Conclusions: Our data demonstrate a previously-unsuspected role for Akirin2 in early cortical development and, given its known nuclear roles, suggest that it may act to regulate gene expression patterns critical for early progenitor cell behavior and cortical neuron production.
Keywords: Apoptosis; Cortical development; Dorsal telencephalon; Microcephaly; Neural progenitor; Neuronal differentiation.
Figures


Similar articles
-
A critical role for the nuclear protein Akirin2 in the formation of mammalian muscle in vivo.Genesis. 2019 May;57(5):e23286. doi: 10.1002/dvg.23286. Epub 2019 Mar 12. Genesis. 2019. PMID: 30801883
-
The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice.Development. 1998 Jun;125(12):2315-25. doi: 10.1242/dev.125.12.2315. Development. 1998. PMID: 9584130
-
Essential Function for the Nuclear Protein Akirin2 in B Cell Activation and Humoral Immune Responses.J Immunol. 2015 Jul 15;195(2):519-27. doi: 10.4049/jimmunol.1500373. Epub 2015 Jun 3. J Immunol. 2015. PMID: 26041538
-
Regulation of brain development and brain function by the transcriptional repressor RP58.Brain Res. 2019 Feb 15;1705:15-23. doi: 10.1016/j.brainres.2018.02.042. Epub 2018 Mar 1. Brain Res. 2019. PMID: 29501651 Review.
-
Extra-cell cycle regulatory functions of cyclin-dependent kinases (CDK) and CDK inhibitor proteins contribute to brain development and neurological disorders.Genes Cells. 2013 Mar;18(3):176-94. doi: 10.1111/gtc.12029. Epub 2013 Jan 7. Genes Cells. 2013. PMID: 23294285 Free PMC article. Review.
Cited by
-
p53-mediated neurodegeneration in the absence of the nuclear protein Akirin2.iScience. 2022 Jan 25;25(2):103814. doi: 10.1016/j.isci.2022.103814. eCollection 2022 Feb 18. iScience. 2022. PMID: 35198879 Free PMC article.
-
Akirin proteins in development and disease: critical roles and mechanisms of action.Cell Mol Life Sci. 2020 Nov;77(21):4237-4254. doi: 10.1007/s00018-020-03531-w. Epub 2020 May 2. Cell Mol Life Sci. 2020. PMID: 32361777 Free PMC article. Review.
-
Akirin Is Required for Muscle Function and Acts Through the TGF-β Sma/Mab Signaling Pathway in Caenorhabditis elegans Development.G3 (Bethesda). 2020 Jan 7;10(1):387-400. doi: 10.1534/g3.119.400377. G3 (Bethesda). 2020. PMID: 31767636 Free PMC article.
-
Function of cofactor Akirin2 in the regulation of gene expression in model human Caucasian neutrophil-like HL60 cells.Biosci Rep. 2021 Jul 30;41(7):BSR20211120. doi: 10.1042/BSR20211120. Biosci Rep. 2021. PMID: 34291801 Free PMC article.
-
The phenotypic spectrum of proximal 6q deletions based on a large cohort derived from social media and literature reports.Eur J Hum Genet. 2018 Oct;26(10):1478-1489. doi: 10.1038/s41431-018-0172-9. Epub 2018 Jun 8. Eur J Hum Genet. 2018. PMID: 29904178 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

