Results of a Doravirine-Atorvastatin Drug-Drug Interaction Study
- PMID: 27872071
- PMCID: PMC5278756
- DOI: 10.1128/AAC.01364-16
Results of a Doravirine-Atorvastatin Drug-Drug Interaction Study
Abstract
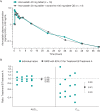
Doravirine is a novel, highly potent, nonnucleoside reverse transcriptase inhibitor that is administered once daily and that is in development for the treatment of HIV-1 infection. In vitro and clinical data suggest that doravirine is unlikely to cause significant drug-drug interactions via major drug-metabolizing enzymes or transporters. As a common HIV-1 infection comorbidity, hypercholesterolemia is often treated with statins, including the commonly prescribed atorvastatin. Atorvastatin is subject to drug-drug interactions with cytochrome P450 3A4 (CYP3A4) inhibitors. Increased exposure due to CYP3A4 inhibition may lead to serious adverse events (AEs), including rhabdomyolysis. Furthermore, atorvastatin is a substrate for breast cancer resistance protein (BCRP), of which doravirine may be a weak inhibitor; this may increase atorvastatin exposure. The potential of doravirine to affect atorvastatin pharmacokinetics was investigated in a two-period, fixed-sequence study in healthy individuals. In period 1, a single dose of atorvastatin at 20 mg was administered followed by a 72-h washout. In period 2, doravirine at 100 mg was administered once daily for 8 days, with a single dose of atorvastatin at 20 mg concomitantly being administered on day 5. Sixteen subjects were enrolled, and 14 completed the trial; 2 discontinued due to AEs unrelated to the treatment. The atorvastatin area under the curve from time zero to infinity was similar with and without doravirine (geometric mean ratio [GMR] for doravirine-atorvastatin/atorvastatin, 0.98; 90% confidence interval [CI], 0.90 to 1.06), while the maximum concentration decreased by 33% (GMR for doravirine-atorvastatin/atorvastatin, 0.67; 90% CI, 0.52 to 0.85). These changes were deemed not to be clinically meaningful. Both of the study drugs were generally well tolerated. Doravirine had no clinically relevant effect on atorvastatin pharmacokinetics in healthy subjects, providing support for the coadministration of doravirine and atorvastatin.
Keywords: CYP3A4; atorvastatin; doravirine; human immunodeficiency virus; hypercholesterolemia; pharmacokinetics.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Anderson MS, Gilmartin J, Cilissen C, De Lepeleire I, van Bortel L, Dockendorf MF, Tetteh E, Ancona JK, Liu R, Guo Y, Wagner JA, Butterton JR. 2015. Safety, tolerability and pharmacokinetics of doravirine, a novel HIV non-nucleoside reverse transcriptase inhibitor, after single and multiple doses in healthy subjects. Antivir Ther 20:397–405. - PubMed
-
- Gatell JM, Morales-Ramirez JO, Hagins DP, Thompson M, Keikawus A, Hoffmann C, Rugina S, Osiyemi O, Escoriu S, Dretler R, Harvey C, Xu X, Teppler H. 2014. Forty-eight-week efficacy and safety and early CNS tolerability of doravirine (MK-1439), a novel NNRTI, with TDF/FTC in ART-naive HIV-positive patients. J Int AIDS Soc 17(4 Suppl 3):19532. - PMC - PubMed
-
- Gatell JM, Raffi F, Plettenberg A, Smith D, Portilla J, Hoffmann C, Arasteh K, Thompson M, Hagins DP, Morales-Ramirez JO, Xu X, Teppler H. 2015. Efficacy and safety of doravirine 100mg QD versus efavirenz 600mg QD with TDF/FTC in ART-naive HIV-infected patients: week 24 results, abstr TUAB0104. Abstr 8th Int AIDS Soc Conf HIV Pathog Treatment Prev.
-
- World Health Organization. 2015. WHO model list of essential medicines, 19th list World Health Organization, Geneva, Switzerland: http://www.who.int/medicines/publications/essentialmedicines/en/.
-
- Sanchez RI, Fillgrove K, Hafey M, Palamanda J, Newton D, Lu B, Bleasby K. 2015. In vitro evaluation of doravirine potential for pharmacokinetic drug interactions, abstr P95. Abstr 20th North Am Meet Int Soc Study Xenobiotics (ISSX), Orlando, FL.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

