Macrophage-Secreted TNFα and TGFβ1 Influence Migration Speed and Persistence of Cancer Cells in 3D Tissue Culture via Independent Pathways
- PMID: 27872091
- PMCID: PMC5243269
- DOI: 10.1158/0008-5472.CAN-16-0442
Macrophage-Secreted TNFα and TGFβ1 Influence Migration Speed and Persistence of Cancer Cells in 3D Tissue Culture via Independent Pathways
Abstract
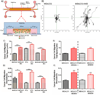
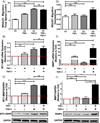
The ability of a cancer cell to migrate through the dense extracellular matrix within and surrounding the solid tumor is a critical determinant of metastasis. Macrophages enhance invasion and metastasis in the tumor microenvironment, but the basis for their effects is not fully understood. Using a microfluidic 3D cell migration assay, we found that the presence of macrophages enhanced the speed and persistence of cancer cell migration through a 3D extracellular matrix in a matrix metalloproteinases (MMP)-dependent fashion. Mechanistic investigations revealed that macrophage-released TNFα and TGFβ1 mediated the observed behaviors by two distinct pathways. These factors synergistically enhanced migration persistence through a synergistic induction of NF-κB-dependent MMP1 expression in cancer cells. In contrast, macrophage-released TGFβ1 enhanced migration speed primarily by inducing MT1-MMP expression. Taken together, our results reveal new insights into how macrophages enhance cancer cell metastasis, and they identify TNFα and TGFβ1 dual blockade as an antimetastatic strategy in solid tumors. Cancer Res; 77(2); 279-90. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
The authors disclose no potential conflict of interest.
Figures
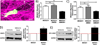
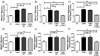



References
-
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–5596. - PubMed
-
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. - PubMed
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

