An oligotrophic deep-subsurface community dependent on syntrophy is dominated by sulfur-driven autotrophic denitrifiers
- PMID: 27872277
- PMCID: PMC5150411
- DOI: 10.1073/pnas.1612244113
An oligotrophic deep-subsurface community dependent on syntrophy is dominated by sulfur-driven autotrophic denitrifiers
Abstract
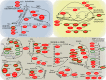
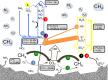
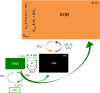
Subsurface lithoautotrophic microbial ecosystems (SLiMEs) under oligotrophic conditions are typically supported by H2 Methanogens and sulfate reducers, and the respective energy processes, are thought to be the dominant players and have been the research foci. Recent investigations showed that, in some deep, fluid-filled fractures in the Witwatersrand Basin, South Africa, methanogens contribute <5% of the total DNA and appear to produce sufficient CH4 to support the rest of the diverse community. This paradoxical situation reflects our lack of knowledge about the in situ metabolic diversity and the overall ecological trophic structure of SLiMEs. Here, we show the active metabolic processes and interactions in one of these communities by combining metatranscriptomic assemblies, metaproteomic and stable isotopic data, and thermodynamic modeling. Dominating the active community are four autotrophic β-proteobacterial genera that are capable of oxidizing sulfur by denitrification, a process that was previously unnoticed in the deep subsurface. They co-occur with sulfate reducers, anaerobic methane oxidizers, and methanogens, which each comprise <5% of the total community. Syntrophic interactions between these microbial groups remove thermodynamic bottlenecks and enable diverse metabolic reactions to occur under the oligotrophic conditions that dominate in the subsurface. The dominance of sulfur oxidizers is explained by the availability of electron donors and acceptors to these microorganisms and the ability of sulfur-oxidizing denitrifiers to gain energy through concomitant S and H2 oxidation. We demonstrate that SLiMEs support taxonomically and metabolically diverse microorganisms, which, through developing syntrophic partnerships, overcome thermodynamic barriers imposed by the environmental conditions in the deep subsurface.
Keywords: active subsurface environment; inverted biomass pyramid; metabolic interactions; sulfur-driven autotrophic denitrifiers; syntrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Stevens TO, McKinley JP. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science. 1995;270:450–454.
-
- Lin L-H, et al. Long-term sustainability of a high-energy, low-diversity crustal biome. Science. 2006;314(5798):479–482. - PubMed
-
- Lever MA, et al. Evidence for microbial carbon and sulfur cycling in deeply buried ridge flank basalt. Science. 2013;339(6125):1305–1308. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

