Atomistic insight into lipid translocation by a TMEM16 scramblase
- PMID: 27872308
- PMCID: PMC5150362
- DOI: 10.1073/pnas.1607574113
Atomistic insight into lipid translocation by a TMEM16 scramblase
Abstract
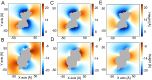
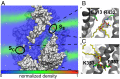
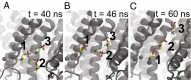
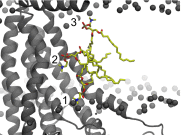
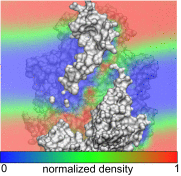
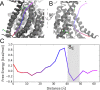
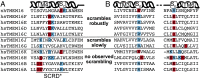
The transmembrane protein 16 (TMEM16) family of membrane proteins includes both lipid scramblases and ion channels involved in olfaction, nociception, and blood coagulation. The crystal structure of the fungal Nectria haematococca TMEM16 (nhTMEM16) scramblase suggested a putative mechanism of lipid transport, whereby polar and charged lipid headgroups move through the low-dielectric environment of the membrane by traversing a hydrophilic groove on the membrane-spanning surface of the protein. Here, we use computational methods to explore the membrane-protein interactions involved in lipid scrambling. Fast, continuum membrane-bending calculations reveal a global pattern of charged and hydrophobic surface residues that bends the membrane in a large-amplitude sinusoidal wave, resulting in bilayer thinning across the hydrophilic groove. Atomic simulations uncover two lipid headgroup-interaction sites flanking the groove. The cytoplasmic site nucleates headgroup-dipole stacking interactions that form a chain of lipid molecules that penetrate into the groove. In two instances, a cytoplasmic lipid interdigitates into this chain, crosses the bilayer, and enters the extracellular leaflet, and the reverse process happens twice as well. Continuum membrane-bending analysis carried out on homology models of mammalian homologs shows that these family members also bend the membrane-even those that lack scramblase activity. Sequence alignments show that the lipid-interaction sites are conserved in many family members but less so in those with reduced scrambling ability. Our analysis provides insight into how large-scale membrane bending and protein chemistry facilitate lipid permeation in the TMEM16 family, and we hypothesize that membrane interactions also affect ion permeation.
Keywords: TMEM16; anoctamin; continuum membrane models; lipid scrambling; simulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Bevers EM, Rosing J, Zwaal RFA. Development of procoagulant binding sites on the platelet surface. Adv Exp Med Biol. 1985;192:359–371. - PubMed
-
- Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455(7217):1210–1215. - PubMed
-
- Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

