Multifunctional Thioredoxin-Like Protein from the Gastrointestinal Parasitic Nematodes Strongyloides ratti and Trichuris suis Affects Mucosal Homeostasis
- PMID: 27872753
- PMCID: PMC5107843
- DOI: 10.1155/2016/8421597
Multifunctional Thioredoxin-Like Protein from the Gastrointestinal Parasitic Nematodes Strongyloides ratti and Trichuris suis Affects Mucosal Homeostasis
Abstract
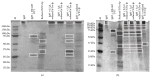
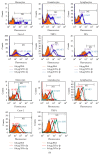
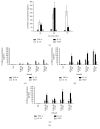
The cellular redox state is important for the regulation of multiple functions and is essential for the maintenance of cellular homeostasis and antioxidant defense. In the excretory/secretory (E/S) products of Strongyloides ratti and Trichuris suis sequences for thioredoxin (Trx) and Trx-like protein (Trx-lp) were identified. To characterize the antioxidant Trx-lp and its interaction with the parasite's mucosal habitat, S. ratti and T. suis Trx-lps were cloned and recombinantly expressed. The primary antioxidative activity was assured by reduction of insulin and IgM. Further analysis applying an in vitro mucosal 3D-cell culture model revealed that the secreted Trx-lps were able to bind to monocytic and intestinal epithelial cells and induce the time-dependent release of cytokines such as TNF-α, IL-22, and TSLP. In addition, the redox proteins also possessed chemotactic activity for monocytic THP-1 cells and fostered epithelial wound healing activity. These results confirm that the parasite-secreted Trx-lps are multifunctional proteins that can affect the host intestinal mucosa.
Figures






Similar articles
-
SPARC (secreted protein acidic and rich in cysteine) of the intestinal nematode Strongyloides ratti is involved in mucosa-associated parasite-host interaction.Mol Biochem Parasitol. 2016 Jun;207(2):75-83. doi: 10.1016/j.molbiopara.2016.06.001. Epub 2016 Jun 3. Mol Biochem Parasitol. 2016. PMID: 27268729
-
Comparative characterization of two galectins excreted-secreted from intestine-dwelling parasitic versus free-living females of the soil-transmitted nematode Strongyloides.Mol Biochem Parasitol. 2018 Oct;225:73-83. doi: 10.1016/j.molbiopara.2018.08.008. Epub 2018 Sep 1. Mol Biochem Parasitol. 2018. PMID: 30179636
-
Stage-specific excretory-secretory small heat shock proteins from the parasitic nematode Strongyloides ratti--putative links to host's intestinal mucosal defense system.FEBS J. 2011 Sep;278(18):3319-36. doi: 10.1111/j.1742-4658.2011.08248.x. Epub 2011 Aug 24. FEBS J. 2011. PMID: 21762402 Free PMC article.
-
The thioredoxin antioxidant system.Free Radic Biol Med. 2014 Jan;66:75-87. doi: 10.1016/j.freeradbiomed.2013.07.036. Epub 2013 Jul 27. Free Radic Biol Med. 2014. PMID: 23899494 Review.
-
Redox regulation by thioredoxin superfamily; protection against oxidative stress and aging.Free Radic Res. 2000 Dec;33(6):851-5. doi: 10.1080/10715760000301361. Free Radic Res. 2000. PMID: 11237106 Review.
Cited by
-
Onchocerca - infected cattle produce strong antibody responses to excretory-secretory proteins released from adult male Onchocerca ochengi worms.BMC Infect Dis. 2018 May 2;18(1):200. doi: 10.1186/s12879-018-3109-6. BMC Infect Dis. 2018. PMID: 29716541 Free PMC article.
-
Development of a Multiplex Bead Assay for the Detection of IgG Antibody Responses to Guinea Worm.Am J Trop Med Hyg. 2020 Dec;103(6):2294-2304. doi: 10.4269/ajtmh.20-0511. Epub 2020 Sep 3. Am J Trop Med Hyg. 2020. PMID: 32901602 Free PMC article.
-
Strongyloides ratti infection in mice: immune response and immune modulation.Philos Trans R Soc Lond B Biol Sci. 2024 Jan 15;379(1894):20220440. doi: 10.1098/rstb.2022.0440. Epub 2023 Nov 27. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38008111 Free PMC article. Review.
-
Cloning, Functional Characterization and Response to Cadmium Stress of the Thioredoxin-like Protein 1 Gene from Phascolosoma esculenta.Int J Mol Sci. 2021 Dec 29;23(1):332. doi: 10.3390/ijms23010332. Int J Mol Sci. 2021. PMID: 35008758 Free PMC article.
-
Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host-parasite communication.J Extracell Vesicles. 2018 Jan 21;7(1):1428004. doi: 10.1080/20013078.2018.1428004. eCollection 2018. J Extracell Vesicles. 2018. PMID: 29410780 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

