Abnormal visuomotor processing in schizophrenia
- PMID: 27872809
- PMCID: PMC5107643
- DOI: 10.1016/j.nicl.2015.08.005
Abnormal visuomotor processing in schizophrenia
Abstract
Subtle disturbances of visual and motor function are known features of schizophrenia and can greatly impact quality of life; however, few studies investigate these abnormalities using simple visuomotor stimuli. In healthy people, electrophysiological data show that beta band oscillations in sensorimotor cortex decrease during movement execution (event-related beta desynchronisation (ERBD)), then increase above baseline for a short time after the movement (post-movement beta rebound (PMBR)); whilst in visual cortex, gamma oscillations are increased throughout stimulus presentation. In this study, we used a self-paced visuomotor paradigm and magnetoencephalography (MEG) to contrast these responses in patients with schizophrenia and control volunteers. We found significant reductions in the peak-to-peak change in amplitude from ERBD to PMBR in schizophrenia compared with controls. This effect was strongest in patients who made fewer movements, whereas beta was not modulated by movement in controls. There was no significant difference in the amplitude of visual gamma between patients and controls. These data demonstrate that clear abnormalities in basic sensorimotor processing in schizophrenia can be observed using a very simple MEG paradigm.
Keywords: Electrophysiological processes; Magnetoencephalography; Motor cortex; Schizophrenia; Visual cortex.
Figures
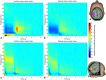
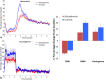
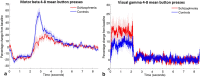
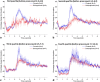
References
-
- Anderson P.M., Pinault D., O’Brien T.J., Jones N.C. Chronic administration of antipsychotics attenuates ongoing and ketamine-induced increases in cortical gamma oscillations. Int. J. Neuropsychopharm. 2014;17(11):1895–1904. - PubMed
-
- APA . fourth edition. American Psychiatric Association; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders.
-
- Bodén R., Abrahamsson T., Holm G., Borg J. Psychomotor and cognitive deficits as predictors of 5-year outcome in first-episode schizophrenia. Nord. J. Psychiatry. 2014;68(4):282–288. - PubMed
-
- Bombin I., Arango C., Buchanan R.W. Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr. Bull. 2005;31(4):962–977. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

