Oxygenation of the Intraportally Transplanted Pancreatic Islet
- PMID: 27872862
- PMCID: PMC5107248
- DOI: 10.1155/2016/7625947
Oxygenation of the Intraportally Transplanted Pancreatic Islet
Abstract
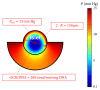
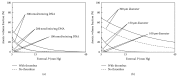
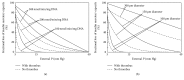
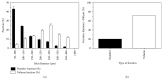
Intraportal islet transplantation (IT) is not widely utilized as a treatment for type 1 diabetes. Oxygenation of the intraportally transplanted islet has not been studied extensively. We present a diffusion-reaction model that predicts the presence of an anoxic core and a larger partly functional core within intraportally transplanted islets. Four variables were studied: islet diameter, islet fractional viability, external oxygen partial pressure (P) (in surrounding portal blood), and presence or absence of a thrombus on the islet surface. Results indicate that an islet with average size and fractional viability exhibits an anoxic volume fraction (AVF) of 14% and a function loss of 72% at a low external P. Thrombus formation increased AVF to 30% and function loss to 92%, suggesting that the effect of thrombosis may be substantial. External P and islet diameter accounted for the greatest overall impact on AVF and loss of function. At our institutions, large human alloislets (>200 μm diameter) account for ~20% of total islet number but ~70% of total islet volume; since most of the total transplanted islet volume is accounted for by large islets, most of the intraportal islet cells are likely to be anoxic and not fully functional.
Figures




Similar articles
-
Intraportal islet oxygenation.J Diabetes Sci Technol. 2014 May;8(3):575-80. doi: 10.1177/1932296814525827. Epub 2014 Mar 6. J Diabetes Sci Technol. 2014. PMID: 24876622 Free PMC article. Review.
-
Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin.Transplantation. 2000 Mar 15;69(5):711-9. doi: 10.1097/00007890-200003150-00007. Transplantation. 2000. PMID: 10755515
-
Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation.Lancet. 2002 Dec 21-28;360(9350):2039-45. doi: 10.1016/s0140-6736(02)12020-4. Lancet. 2002. PMID: 12504401
-
Results of our first nine intraportal islet allografts in type 1, insulin-dependent diabetic patients.Transplantation. 1991 Jan;51(1):76-85. doi: 10.1097/00007890-199101000-00012. Transplantation. 1991. PMID: 1987709 Clinical Trial.
-
Oxygenation of islets and its role in transplantation.Curr Opin Organ Transplant. 2009 Dec;14(6):688-93. doi: 10.1097/MOT.0b013e32833239ff. Curr Opin Organ Transplant. 2009. PMID: 19745734 Review.
Cited by
-
Preliminary Studies of the Impact of CXCL12 on the Foreign Body Reaction to Pancreatic Islets Microencapsulated in Alginate in Nonhuman Primates.Transplant Direct. 2019 Apr 15;5(5):e447. doi: 10.1097/TXD.0000000000000890. eCollection 2019 May. Transplant Direct. 2019. PMID: 31165082 Free PMC article.
-
Modeling of a Bioengineered Immunomodulating Microenvironment for Cell Therapy.Adv Healthc Mater. 2025 Feb;14(5):e2304003. doi: 10.1002/adhm.202304003. Epub 2024 Jan 20. Adv Healthc Mater. 2025. PMID: 38215451 Free PMC article.
-
An inverse-breathing encapsulation system for cell delivery.Sci Adv. 2021 May 14;7(20):eabd5835. doi: 10.1126/sciadv.abd5835. Print 2021 May. Sci Adv. 2021. PMID: 33990318 Free PMC article.
-
Impact of Oxygen on Pancreatic Islet Survival.Pancreas. 2018 May/Jun;47(5):533-543. doi: 10.1097/MPA.0000000000001050. Pancreas. 2018. PMID: 29621044 Free PMC article. Review.
-
Insulinoma-derived pseudo-islets for diabetes research.Am J Physiol Cell Physiol. 2021 Aug 1;321(2):C247-C256. doi: 10.1152/ajpcell.00466.2020. Epub 2021 Jun 9. Am J Physiol Cell Physiol. 2021. PMID: 34106785 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

