Evaluation of the drug solubility and rush ageing on drug release performance of various model drugs from the modified release polyethylene oxide matrix tablets
- PMID: 27873080
- PMCID: PMC5222914
- DOI: 10.1007/s13346-016-0344-5
Evaluation of the drug solubility and rush ageing on drug release performance of various model drugs from the modified release polyethylene oxide matrix tablets
Erratum in
-
Correction to: Evaluation of the drug solubility and rush ageing on drug release performance of various model drugs from the modified release polyethylene oxide matrix tablets.Drug Deliv Transl Res. 2023 Feb;13(2):702-703. doi: 10.1007/s13346-022-01240-4. Drug Deliv Transl Res. 2023. PMID: 36121614 Free PMC article. No abstract available.
Abstract
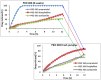
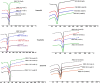
Hydrophilic matrix systems are currently some of the most widely used drug delivery systems for controlled-release oral dosage forms. Amongst a variety of polymers, polyethylene oxide (PEO) is considered an important material used in pharmaceutical formulations. As PEO is sensitive to thermal oxidation, it is susceptible to free radical oxidative attack. The aim of this study was to investigate the stability of PEO based formulations containing different model drugs with different water solubility, namely propranolol HCl, theophylline and zonisamide. Both polyox matrices 750 and 303 grade were used as model carriers for the manufacture of tablets stored at 40 °C. The results of the present study suggest that the drug release from the matrix was affected by the length of storage conditions, solubility of drugs and the molecular weight of the polymers. Generally, increased drug release rates were prevalent in soluble drug formulations (propranolol) when stored at the elevated temperature (40 °C). In contrast, it was not observed with semi soluble (theophylline) and poorly soluble (zonisamide) drugs especially when formulated with PEO 303 polymer. This indicates that the main parameters controlling the drug release from fresh polyox matrices are the solubility of the drug in the dissolution medium and the molecular weight of the polymer. DSC traces indicated that that there was a big difference in the enthalpy and melting points of fresh and aged PEO samples containing propranolol, whereas the melting point of the aged polyox samples containing theophylline and zonisamide was unaffected. Graphical abstract ᅟ.
Keywords: Controlled release; Drug release; Drug solubility; Polyox matrices; Stability.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
-
- Haan PD, Lerk CF. Oral controlled release dosage forms: a review. Pharm World Sci. 1984;6:57–67. - PubMed
-
- Khan MA, Karnachi AA, Singh SK, Sastry SV, Kislalioglu SM, Bolto S. Controlled release coprecipitates: formulation considerations. J Con Rel. 1995;37:132–141. doi: 10.1016/0168-3659(95)00073-H. - DOI
-
- Rane M, Parmar J, Siahboomi AR. Hydrophilic matrices for oral extended release: influence of fillers on drug release from HPMC matrices. Pharma Times. 2010;42:41–45.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

