A Comparison of Pharmacokinetic and Pharmacodynamic Properties Between Faster-Acting Insulin Aspart and Insulin Aspart in Elderly Subjects with Type 1 Diabetes Mellitus
- PMID: 27873152
- PMCID: PMC5222895
- DOI: 10.1007/s40266-016-0418-6
A Comparison of Pharmacokinetic and Pharmacodynamic Properties Between Faster-Acting Insulin Aspart and Insulin Aspart in Elderly Subjects with Type 1 Diabetes Mellitus
Abstract
Background: Due to population aging, an increasing number of elderly patients with diabetes use insulin. It is therefore important to investigate the characteristics of new insulins in this population. Faster-acting insulin aspart (faster aspart) is insulin aspart (IAsp) in a new formulation with faster absorption. This study investigated the pharmacological properties of faster aspart in elderly subjects with type 1 diabetes mellitus (T1DM).
Methods: In a randomised, double-blind, two-period crossover trial, 30 elderly (≥65 years) and 37 younger adults (18-35 years) with T1DM received single subcutaneous faster aspart or IAsp dosing (0.2 U/kg) and underwent an euglycaemic clamp (target 5.5 mmol/L) for up to 12 h.
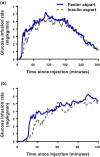
Results: The pharmacokinetic and pharmacodynamic time profiles were left-shifted for faster aspart versus IAsp. In each age group, onset of appearance occurred approximately twice as fast (~3 min earlier) and early exposure (area under the concentration-time curve [AUC] for serum IAsp from time zero to 30 min [AUCIAsp,0-30 min]) was greater (by 86% in elderly and 67% in younger adults) for faster aspart than for IAsp. Likewise, onset of action occurred 10 min faster in the elderly and 9 min faster in younger adults, and early glucose-lowering effect (AUC for the glucose infusion rate [GIR] from time zero to 30 min [AUCGIR,0-30 min]) was greater (by 109%) for faster aspart than for IAsp in both age groups. Total exposure (AUCIAsp,0-t) and the maximum concentration (C max) for faster aspart were greater (by 30 and 28%, respectively) in elderly than in younger adults. No age group differences were seen for the total (AUCGIR,0-t) or maximum (GIRmax) glucose-lowering effect.
Conclusion: This study demonstrated that the ultra-fast pharmacological properties of faster aspart are similar in elderly subjects and younger adults with T1DM. ClinicalTrials.gov Identifier: NCT02003677.
Conflict of interest statement
Compliance with Ethical StandardsAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.FundingThis study was funded by Novo Nordisk.Conflict of interestTim Heise is a shareholder of Profil, which has received research funds from Adocia, AstraZeneca, Becton Dickinson, Biocon, Boehringer Ingelheim, Dance Biopharm, Eli Lilly, Grünenthal, Gulf Pharmaceutical Industries, Johnson & Johnson, Marvel, MedImmune, Medtronic, Novartis, Novo Nordisk, Roche Diagnostics, Sanofi, Senseonics and Zealand Pharma. In addition, Tim Heise is a member of advisory panels for Novo Nordisk and received speaker honoraria and travel grants from Eli Lilly, Mylan and Novo Nordisk. Kirstine Stender-Petersen, Jacob Bonde Jacobsen and Hanne Haahr are employees and shareholders of Novo Nordisk. Eric Zijlstra received travel grants from Dance Biopharm and Novo Nordisk and speaker honoraria from Novo Nordisk and Roche Diabetes Care. Ulrike Hövelmann declares no conflicts of interest.
Figures


References
-
- US Food and Drug Administration. Inactive ingredient search for approved drug products. http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm. Accessed 7 Oct 2016.
-
- Heise T, Stender-Petersen K, Hövelmann U, et al. Pharmacokinetic and pharmacodynamic properties of faster-acting insulin aspart versus insulin aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clinical Pharmacokinet. doi:10.1007/s40262-016-0473-5. - PMC - PubMed
-
- Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. Faster onset and greater early exposure and glucose-lowering effect with faster-acting insulin aspart vs. insulin aspart: a pooled analysis in subjects with type 1 diabetes [abstract]. Diabetes. 2016;65(Suppl. 1):A239.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

