Decitabine as a First-Line Treatment for Older Adults Newly Diagnosed with Acute Myeloid Leukemia
- PMID: 27873493
- PMCID: PMC5122650
- DOI: 10.3349/ymj.2017.58.1.35
Decitabine as a First-Line Treatment for Older Adults Newly Diagnosed with Acute Myeloid Leukemia
Abstract
Purpose: Decitabine, a DNA hypomethylating agent, was recently approved for use in Korea for older adults with acute myeloid leukemia (AML) who are not candidates for standard chemotherapy. This study aimed to evaluate the role of decitabine as a first-line treatment for older adults with AML.
Materials and methods: Twenty-four patients with AML who received at least one course of decitabine (20 mg/m²/d intravenously for 5 days every 4 weeks) as a first-line therapy at Severance Hospital were evaluated retrospectively.
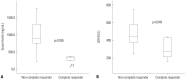
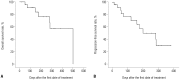
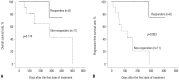
Results: The median age of the patients was 73.5 years. The longest follow-up duration was 502 days. A total of 113 cycles of treatment were given to 24 patients, and the median number of cycles was four (range, 1-14). Thirteen patients dropped out because of death, no or loss of response, patient refusal, or transfer to another hospital. Twenty-one (87.5%) and 12 (50%) patients completed the second and fourth cycles, respectively, and responses to treatment were evaluated in 17. A complete response (CR) or CR with incomplete blood-count recovery was achieved in six (35.3%) patients, and the estimated median overall survival was 502 days. Ten patients developed grade >2 hematologic or non-hematologic toxicities. In univariate analysis, bone marrow blasts, lactate dehydrogenase, serum ferritin level, and bone marrow iron were significantly associated with response to decitabine.
Conclusion: Five-day decitabine treatment showed acceptable efficacy in older patients with AML who are unfit for conventional chemotherapy, with a CR rate 35.3% and about a median overall survival of 18 months.
Keywords: AML; Decitabine; elderly; treatment.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

References
-
- Korea Central Cancer Registry NCC. Annual report of cancer statistics in Korea in 2012. Sejong: Ministry of Health and Welfare; 2014.
-
- Sorm F, Veselý J. Effect of 5-aza-2'-deoxycytidine against leukemic and hemopoietic tissues in AKR mice. Neoplasma. 1968;15:339–343. - PubMed
-
- Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–1640. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

