A Voltammetric Biosensor Based on Glassy Carbon Electrodes Modified with Single-Walled Carbon Nanotubes/Hemoglobin for Detection of Acrylamide in Water Extracts from Potato Crisps
- PMID: 27873843
- PMCID: PMC3705533
- DOI: 10.3390/s8095832
A Voltammetric Biosensor Based on Glassy Carbon Electrodes Modified with Single-Walled Carbon Nanotubes/Hemoglobin for Detection of Acrylamide in Water Extracts from Potato Crisps
Abstract
The presence of toxic acrylamide in a wide range of food products such as potato crisps, French fries or bread has been confirmed by Swedish scientists from Stockholm University. The neurotoxicity, possible carcinogenicity of this compound and its metabolites compels us to control them by quantitative and qualitative assays. Acrylamide forms adduct with hemoglobin (Hb) as a result of the reaction the -NH2 group of the Nterminal valine with acrylamide. In this work we present the use of glassy carbon electrodes coated with single-walled carbon nanotubes (SWCNTs) and Hb for voltammetric detection of acrylamide in water solutions. The electrodes presented a very low detection limit (1.0×10-9 M). The validation made in the matrix obtained by water extraction of potato crisps showed that the electrodes presented are suitable for the direct determination of acrylamide in food samples.
Keywords: Acrylamide; OSWV; SWCNTs; hemoglobin; potato crisps.
Figures
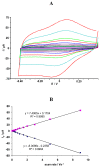

 ) and SWCNT/Hb, Ep,0=44 mV, n=3, 0.9<SD<4.4 (
) and SWCNT/Hb, Ep,0=44 mV, n=3, 0.9<SD<4.4 (
 ).
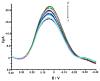
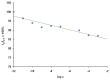
).
References
-
- Tareke E., Rydberg P., Karlsson P., Eriksson S., Törnqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002;50:4998–5006. - PubMed
-
- Ruden C. Acrylamide and cancer risk – export risk assessments and the public debate. Food Chem. Toxicol. 2004;42:335–349. - PubMed
-
- Friedman M. Chemistry, biochemistry and safety of acrylamide. A review. J. Agric. Food Chem. 2003;51:4504–4526. - PubMed
-
- Gökmen V., Şenyuva H.Z., Acar J., Sarıoğlu K. Determination of acrylamide in potato chips and crisps by high-performance liquid chromatography. J. Chrom. A. 2005;1088:193–199. - PubMed
-
- Becalski A., Lau B.P.-Y., Lewis D., Seaman S.W. Acrylamide in foods: occurrence, sources and modelling. J. Agric. Food Chem. 2003;51:802–808. - PubMed
LinkOut - more resources
Full Text Sources

