Fabrication and Optimization of a Nanoporous Platinum Electrode and a Non-enzymatic Glucose Micro-sensor on Silicon
- PMID: 27873863
- PMCID: PMC3707443
- DOI: 10.3390/s8106154
Fabrication and Optimization of a Nanoporous Platinum Electrode and a Non-enzymatic Glucose Micro-sensor on Silicon
Abstract
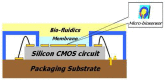
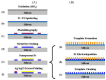
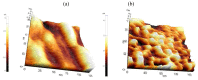
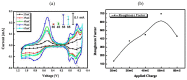
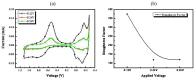
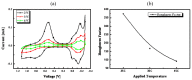
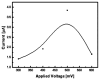
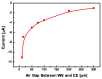
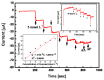
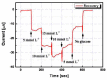
In this paper, optimal conditions for fabrication of nanoporous platinum (Pt) were investigated in order to use it as a sensitive sensing electrode for silicon CMOS integrable non-enzymatic glucose micro-sensor applications. Applied charges, voltages, and temperatures were varied during the electroplating of Pt into the formed nonionic surfactant C16EO8 nano-scaled molds in order to fabricate nanoporous Pt electrodes with large surface roughness factor (RF), uniformity, and reproducibility. The fabricated nanoporous Pt electrodes were characterized using atomic force microscopy (AFM) and electrochemical cyclic voltammograms. Optimal electroplating conditions were determined to be an applied charge of 35 mC/mm2, a voltage of -0.12 V, and a temperature of 25 °C, respectively. The optimized nanoporous Pt electrode had an electrochemical RF of 375 and excellent reproducibility. The optimized nanoporous Pt electrode was applied to fabricate non-enzymatic glucose micro-sensor with three electrode systems. The fabricated sensor had a size of 3 mm x 3 mm, air gap of 10 µm, working electrode (WE) area of 4.4 mm2, and sensitivity of 37.5 µA•L/mmol•cm2. In addition, it showed large detection range from 0.05 to 30 mmolL-1 and stable recovery responsive to the step changes in glucose concentration.
Keywords: Nanoporous platinum; glucose sensor; non-enzymatic; silicon CMOS integrable.
Figures
Similar articles
-
Characterization of electroplated multilayered nanoporous platinum films for electrochemical sensor applications.J Nanosci Nanotechnol. 2013 Oct;13(10):7067-9. doi: 10.1166/jnn.2013.7911. J Nanosci Nanotechnol. 2013. PMID: 24245192
-
Integration of a nanoporous platinum thin film into a microfluidic system for non-enzymatic electrochemical glucose sensing.Anal Sci. 2007 Mar;23(3):277-81. doi: 10.2116/analsci.23.277. Anal Sci. 2007. PMID: 17372368
-
Nanoporous platinum solid-state reference electrode with layer-by-layer polyelectrolyte junction for pH sensing chip.Lab Chip. 2011 Feb 21;11(4):664-71. doi: 10.1039/c0lc00293c. Epub 2010 Dec 7. Lab Chip. 2011. PMID: 21135953
-
Strategies for the fabrication of porous platinum electrodes.Adv Mater. 2011 Nov 16;23(43):4976-5008. doi: 10.1002/adma.201102182. Adv Mater. 2011. PMID: 22180890 Review.
-
Micro/Nano Electrode Array Sensors: Advances in Fabrication and Emerging Applications in Bioanalysis.Front Chem. 2020 Nov 13;8:573865. doi: 10.3389/fchem.2020.573865. eCollection 2020. Front Chem. 2020. PMID: 33324609 Free PMC article. Review.
Cited by
-
Recent Developments and Future Perspective on Electrochemical Glucose Sensors Based on 2D Materials.Biosensors (Basel). 2022 Jun 28;12(7):467. doi: 10.3390/bios12070467. Biosensors (Basel). 2022. PMID: 35884271 Free PMC article. Review.
References
-
- Woderer S., Henninger N., Garthe C.D., Kloetzer H.M., Hajnsek M., Kamecke U., Gretz N. Continuous glucose monitoring in interstitial fluid using glucose oxidase-based sensor compared to established blood glucose measurement in rats. Anal. Chim. Acta. 2007;581:7–12. - PubMed
-
- Kang X.H., Mai Z.B., Zou X.Y., Cai P.X., Mo J.Y. A novel sensitive non-enzymatic glucose sensor. Chin. Chemi. Lett. 2007;18:189–191.
-
- Guan W.J., Li Y., Chen Y.Q., Zhang X.B., Hu G.Q. Glucose biosensor based on multi-wall carbon nanotubes and screen printed carbon electrodes. Biosens. Bioelectron. 2005;21:508–512. - PubMed
-
- Deng C., Li M., Xie Z., Liu M., Tan Y., Xu X., Yao S. New glucose biosensor based on a poly(o-phenylendiamine)/glucose oxidase-glutaraldehyde/Prussian blue/Au electrode with QCM monitoring of various electrode-surface modifications. Anal. Chem. 2006;557:85–94.
-
- Beden B., Largeaud F., Kokoh K.B., Lamy C. Fourier transform infrared reflectance spectroscopic investigation of the electrocatalytic oxidation of D-glucose: identification of reactive intermediates and reaction products. Electrochim. Acta. 1996;41:701–709.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

