The Hippo signalling pathway coordinates organ growth and limits developmental variability by controlling dilp8 expression
- PMID: 27874005
- PMCID: PMC5121414
- DOI: 10.1038/ncomms13505
The Hippo signalling pathway coordinates organ growth and limits developmental variability by controlling dilp8 expression
Abstract
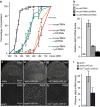
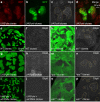
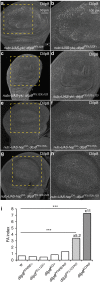
Coordination of organ growth during development is required to generate fit individuals with fixed proportions. We recently identified Drosophila Dilp8 as a key hormone in coupling organ growth with animal maturation. In addition, dilp8 mutant flies exhibit elevated fluctuating asymmetry (FA) demonstrating a function for Dilp8 in ensuring developmental stability. The signals regulating Dilp8 activity during normal development are not yet known. Here, we show that the transcriptional co-activators of the Hippo (Hpo) pathway, Yorkie (Yki, YAP/TAZ) and its DNA-binding partner Scalloped (Sd), directly regulate dilp8 expression through a Hpo-responsive element (HRE) in the dilp8 promoter. We further demonstrate that mutation of the HRE by genome-editing results in animals with increased FA, thereby mimicking full dilp8 loss of function. Therefore, our results indicate that growth coordination of organs is connected to their growth status through a feedback loop involving Hpo and Dilp8 signalling pathways.
Figures

References
-
- Twitty V. C. & Schwind J. L. The growth of eyes and limbs transplanted heteroplastically between two species of Amblystoma. J. Exp. Zool. 59, 61–86 (1931).
-
- Bryant P. J. & Simpson P. Intrinsic and extrinsic control of growth in developing organs. Q. Rev. Biol. 59, 387–415 (1984). - PubMed
-
- Parker N. F. & Shingleton A. W. The coordination of growth among Drosophila organs in response to localized growth-perturbation. Dev. Biol. 357, 318–325 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

