Combinational Immunotherapy with Allo-DRibble Vaccines and Anti-OX40 Co-Stimulation Leads to Generation of Cross-Reactive Effector T Cells and Tumor Regression
- PMID: 27874054
- PMCID: PMC5118714
- DOI: 10.1038/srep37558
Combinational Immunotherapy with Allo-DRibble Vaccines and Anti-OX40 Co-Stimulation Leads to Generation of Cross-Reactive Effector T Cells and Tumor Regression
Abstract
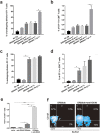
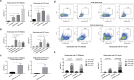
It is well-known that vaccines comprising of irradiated whole tumor cells or tumor-derived heat shock proteins can generate tumor-specific immune responses. In contrast, we showed recently that vaccines composed of autophagosomes (DRibbles) derived from syngeneic sarcomas could induce cross-reactive T-cell responses and cross-protection against the tumor. This unusual property of DRibbles was related to the selective recruitment of defective ribosomal products (DRiPs) and other short-lived proteins (SLiPs) into autophagosomes via sequestosome (SQSTM1, p62) mediated association of ubiquitinated SLiPs to the autophagy gene product LC3. Here, we extend our observations to mammary carcinomas from mice of different genetic background. We demonstrated that combined of intranodal administration of autologous or allogeneic DRibbles together with anti-OX40 antibody led to robust proliferation, expansion, and differentiation of memory and effector T cells. We also showed that SLiPs is an excellent source of antigen for cross-priming of CD8+ T-cells that recognize shared tumor antigens in the context of host MHC class I molecules. Thus, our results provide a strong basis for novel clinical trials that combine allogeneic "off-the-shelf" DRibble vaccines together with antibodies against co-stimulatory molecules.
Conflict of interest statement
Hong-Ming Hu and Bernard A. Fox are co-founders of UbiVac, which has licensed the autophagosome intellectual property. Andrew D. Weinberg and Nicholas P. Morris are founder and employee of AgonOx, which has an ownership interest in OX40 patents. No potential conflicts of interest were disclosed by the other authors.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

