Lymphocytic Microparticles Modulate Angiogenic Properties of Macrophages in Laser-induced Choroidal Neovascularization
- PMID: 27874077
- PMCID: PMC5118818
- DOI: 10.1038/srep37391
Lymphocytic Microparticles Modulate Angiogenic Properties of Macrophages in Laser-induced Choroidal Neovascularization
Abstract
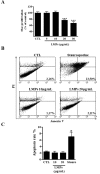
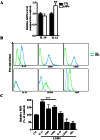
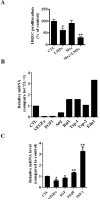
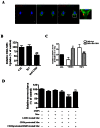
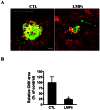
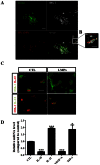
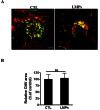
Pathological choroidal neovascularization (CNV) is the common cause of vision loss in patients with age-related macular degeneration (AMD). Macrophages possess potential angiogenic function in CNV. We have demonstrated that human T lymphocyte-derived microparticles (LMPs) exert a potent antiangiogenic effect in several pathological neovascularization models. In this study, we investigated the alteration of proangiogenic properties of macrophages by LMPs treatment in vitro and in vivo models. LMPs regulated the expression of several angiogenesis-related factors in macrophages and consequently stimulated their antiangiogenic effects evidenced by the suppression of the proliferation of human retinal endothelial cells in co-culture experiments. The involvement of CD36 receptor in LMPs uptake by macrophages was demonstrated by in vitro assays and by immunostaining of choroidal flat mounts. In addition, ex vivo experiments showed that CD36 mediates the antiangiogenic effect of LMPs in murine and human choroidal explants. Furthermore, intravitreal injection of LMPs in the mouse model of laser-induced CNV significantly suppressed CNV in CD36 dependent manner. The results of this study suggested an ability of LMPs to alter the gene expression pattern of angiogenesis-related factors in macrophages, which provide important information for a new therapeutic approach for efficiently interfering with both vascular and extravascular components of CNV.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

