Reversible developmental stasis in response to nutrient availability in the Xenopus laevis central nervous system
- PMID: 27875263
- PMCID: PMC5312736
- DOI: 10.1242/jeb.151043
Reversible developmental stasis in response to nutrient availability in the Xenopus laevis central nervous system
Abstract
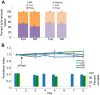
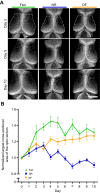
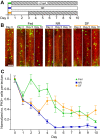
Many organisms confront intermittent nutrient restriction (NR), but the mechanisms to cope with nutrient fluctuations during development are not well understood. This is particularly true of the brain, the development and function of which is energy intensive. Here we examine the effects of nutrient availability on visual system development in Xenopus laevis tadpoles. During the first week of development, tadpoles draw nutrients from maternally provided yolk. Upon yolk depletion, animals forage for food. By altering access to external nutrients after yolk depletion, we identified a period of reversible stasis during tadpole development. We demonstrate that NR results in developmental stasis characterized by a decrease in overall growth of the animals, a failure to progress through developmental stages, and a decrease in volume of the optic tectum. During NR, neural progenitors virtually cease proliferation, but tadpoles swim and behave normally. Introducing food after temporary NR increased neural progenitor cell proliferation more than 10-fold relative to NR tadpoles, and cell proliferation was comparable to that of fed counterparts 1 week after delayed feeding. Delayed feeding also rescued NR-induced body length and tectal volume deficits and partially rescued developmental progression defects. Tadpoles recover from developmental stasis if food is provided within the first 9 days of NR, after which access to food fails to increase cell proliferation. These results show that early stages of tadpole brain development are acutely sensitive to fluctuations in nutrient availability and that NR induces developmental stasis from which animals can recover if food becomes available within a critical window.
Keywords: Development; Nutrition; Optic tectum; Stasis; Xenopus.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



Similar articles
-
Nutrient restriction causes reversible G2 arrest in Xenopus neural progenitors.Development. 2019 Oct 24;146(20):dev178871. doi: 10.1242/dev.178871. Development. 2019. PMID: 31649012 Free PMC article.
-
In vivo time-lapse imaging of cell proliferation and differentiation in the optic tectum of Xenopus laevis tadpoles.J Comp Neurol. 2012 Feb 1;520(2):401-33. doi: 10.1002/cne.22795. J Comp Neurol. 2012. PMID: 22113462 Free PMC article.
-
Thyroid Hormone Acts Locally to Increase Neurogenesis, Neuronal Differentiation, and Dendritic Arbor Elaboration in the Tadpole Visual System.J Neurosci. 2016 Oct 5;36(40):10356-10375. doi: 10.1523/JNEUROSCI.4147-15.2016. J Neurosci. 2016. PMID: 27707971 Free PMC article.
-
Early development and function of the Xenopus tadpole retinotectal circuit.Curr Opin Neurobiol. 2016 Dec;41:17-23. doi: 10.1016/j.conb.2016.07.002. Epub 2016 Jul 29. Curr Opin Neurobiol. 2016. PMID: 27475307 Review.
-
The decision to move: response times, neuronal circuits and sensory memory in a simple vertebrate.Proc Biol Sci. 2019 Mar 27;286(1899):20190297. doi: 10.1098/rspb.2019.0297. Proc Biol Sci. 2019. PMID: 30900536 Free PMC article. Review.
Cited by
-
Phenotypic Switching Resulting From Developmental Plasticity: Fixed or Reversible?Front Physiol. 2020 Jan 22;10:1634. doi: 10.3389/fphys.2019.01634. eCollection 2019. Front Physiol. 2020. PMID: 32038303 Free PMC article. Review.
-
Nutrient availability contributes to a graded refractory period for regeneration in Xenopus tropicalis.Dev Biol. 2021 May;473:59-70. doi: 10.1016/j.ydbio.2021.01.005. Epub 2021 Jan 20. Dev Biol. 2021. PMID: 33484704 Free PMC article.
-
X-Tracker: Automated Analysis of Xenopus Tadpole Visual Avoidance Behavior.bioRxiv [Preprint]. 2024 Oct 12:2024.10.10.617688. doi: 10.1101/2024.10.10.617688. bioRxiv. 2024. PMID: 39416226 Free PMC article. Preprint.
-
An Innate Color Preference Displayed by Xenopus Tadpoles Is Persistent and Requires the Tegmentum.Front Behav Neurosci. 2020 May 12;14:71. doi: 10.3389/fnbeh.2020.00071. eCollection 2020. Front Behav Neurosci. 2020. PMID: 32477078 Free PMC article.
-
Nutrient restriction causes reversible G2 arrest in Xenopus neural progenitors.Development. 2019 Oct 24;146(20):dev178871. doi: 10.1242/dev.178871. Development. 2019. PMID: 31649012 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

