The Fission Yeast Pre-mRNA-processing Factor 18 (prp18+) Has Intron-specific Splicing Functions with Links to G1-S Cell Cycle Progression
- PMID: 27875300
- PMCID: PMC5207164
- DOI: 10.1074/jbc.M116.751289
The Fission Yeast Pre-mRNA-processing Factor 18 (prp18+) Has Intron-specific Splicing Functions with Links to G1-S Cell Cycle Progression
Abstract
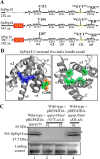
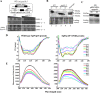
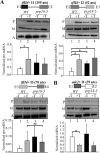
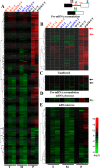
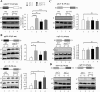
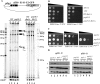
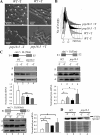
The fission yeast genome, which contains numerous short introns, is an apt model for studies on fungal splicing mechanisms and splicing by intron definition. Here we perform a domain analysis of the evolutionarily conserved Schizosaccharomyces pombe pre-mRNA-processing factor, SpPrp18. Our mutational and biophysical analyses of the C-terminal α-helical bundle reveal critical roles for the conserved region as well as helix five. We generate a novel conditional missense mutant, spprp18-5 To assess the role of SpPrp18, we performed global splicing analyses on cells depleted of prp18+ and the conditional spprp18-5 mutant, which show widespread but intron-specific defects. In the absence of functional SpPrp18, primer extension analyses on a tfIId+ intron 1-containing minitranscript show accumulated pre-mRNA, whereas the lariat intron-exon 2 splicing intermediate was undetectable. These phenotypes also occurred in cells lacking both SpPrp18 and SpDbr1 (lariat debranching enzyme), a genetic background suitable for detection of lariat RNAs. These data indicate a major precatalytic splicing arrest that is corroborated by the genetic interaction between spprp18-5 and spprp2-1, a mutant in the early acting U2AF59 protein. Interestingly, SpPrp18 depletion caused cell cycle arrest before S phase. The compromised splicing of transcripts coding for G1-S regulators, such as Res2, a transcription factor, and Skp1, a regulated proteolysis factor, are shown. The cumulative effects of SpPrp18-dependent intron splicing partly explain the G1 arrest upon the loss of SpPrp18. Our study using conditional depletion of spprp18+ and the spprp18-5 mutant uncovers an intron-specific splicing function and early spliceosomal interactions and suggests links with cell cycle progression.
Keywords: RNA splicing; Schizosaccharomyces pombe; SpPrp18; functional genomics; intron-specific splicing; molecular genetics; mutagenesis; splicing microarrays.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Burge C. B., Tuschl T., and Sharp P. A. (1999) Splicing of precursors to mRNAs by the spliceosomes. in RNA World, 2nd Ed (Gesteland R. F., Cech T. R., and Atkins J. F.) pp. 525–560, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
-
- Will C. L., and Lührmann R. (2006) Spliceosome Structure and Function. in RNA World, 3rd Ed (Gesteland R. F., Cech T. R., and Atkins J. F.) pp. 369–400, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
-
- Horowitz D. S., and Krainer A. R. (1997) A human protein required for the second step of pre-mRNA splicing is functionally related to a yeast splicing factor. Genes Dev. 11, 139–151 - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

