Comparative genomics to explore phylogenetic relationship, cryptic sexual potential and host specificity of Rhynchosporium species on grasses
- PMID: 27875982
- PMCID: PMC5118889
- DOI: 10.1186/s12864-016-3299-5
Comparative genomics to explore phylogenetic relationship, cryptic sexual potential and host specificity of Rhynchosporium species on grasses
Abstract
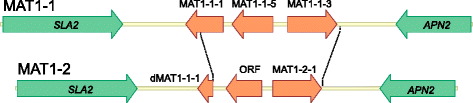
Background: The Rhynchosporium species complex consists of hemibiotrophic fungal pathogens specialized to different sweet grass species including the cereal crops barley and rye. A sexual stage has not been described, but several lines of evidence suggest the occurrence of sexual reproduction. Therefore, a comparative genomics approach was carried out to disclose the evolutionary relationship of the species and to identify genes demonstrating the potential for a sexual cycle. Furthermore, due to the evolutionary very young age of the five species currently known, this genus appears to be well-suited to address the question at the molecular level of how pathogenic fungi adapt to their hosts.
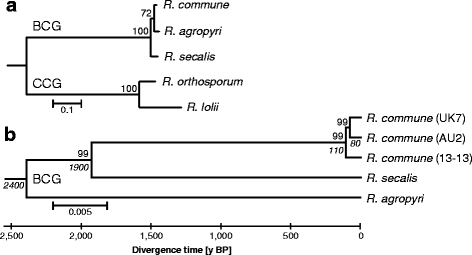
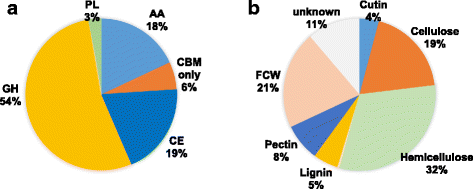
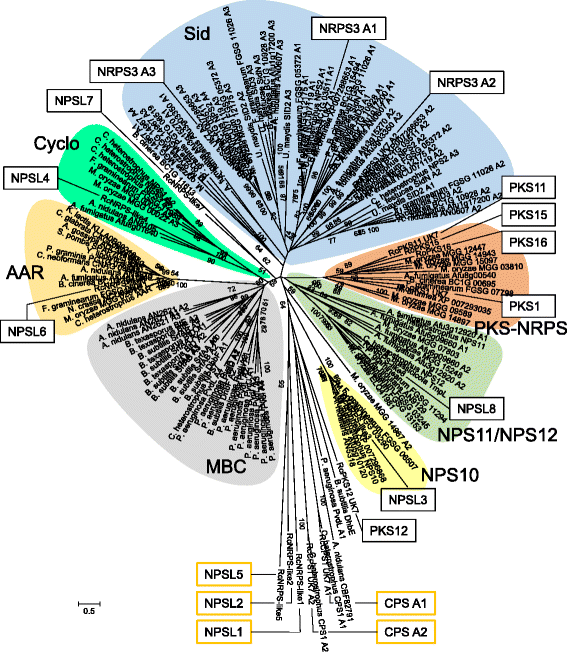
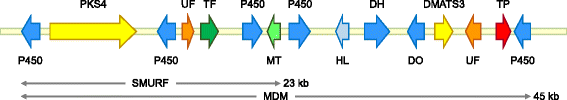
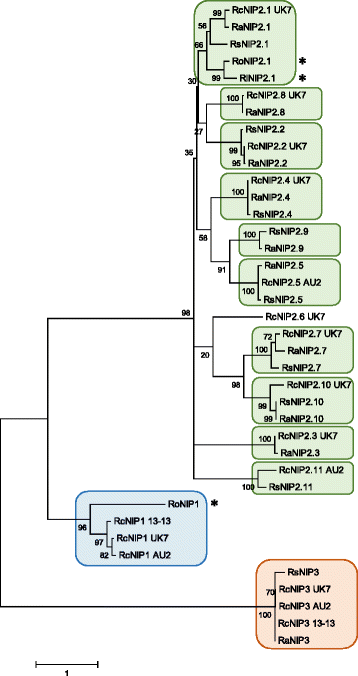
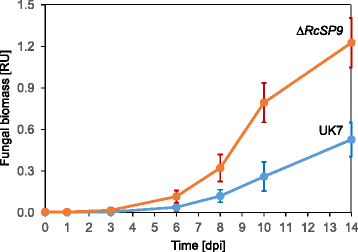
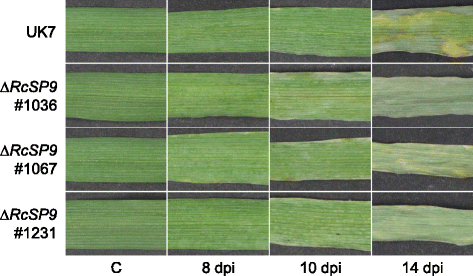
Results: The genomes of the different Rhynchosporium species were sequenced, assembled and annotated using ab initio gene predictors trained on several fungal genomes as well as on Rhynchosporium expressed sequence tags. Structures of the rDNA regions and genome-wide single nucleotide polymorphisms provided a hypothesis for intra-genus evolution. Homology screening detected core meiotic genes along with most genes crucial for sexual recombination in ascomycete fungi. In addition, a large number of cell wall-degrading enzymes that is characteristic for hemibiotrophic and necrotrophic fungi infecting monocotyledonous hosts were found. Furthermore, the Rhynchosporium genomes carry a repertoire of genes coding for polyketide synthases and non-ribosomal peptide synthetases. Several of these genes are missing from the genome of the closest sequenced relative, the poplar pathogen Marssonina brunnea, and are possibly involved in adaptation to the grass hosts. Most importantly, six species-specific genes coding for protein effectors were identified in R. commune. Their deletion yielded mutants that grew more vigorously in planta than the wild type.
Conclusion: Both cryptic sexuality and secondary metabolites may have contributed to host adaptation. Most importantly, however, the growth-retarding activity of the species-specific effectors suggests that host adaptation of R. commune aims at extending the biotrophic stage at the expense of the necrotrophic stage of pathogenesis. Like other apoplastic fungi Rhynchosporium colonizes the intercellular matrix of host leaves relatively slowly without causing symptoms, reminiscent of the development of endophytic fungi. Rhynchosporium may therefore become an object for studying the mutualism-parasitism transition.
Keywords: CAZymes; Effectors; Host specificity; Leotiomycetes; Non-ribosomal peptide synthetases; Phylogenetic evolution; Polyketide synthases; Rhynchosporium; Sex-related genes; Whole genome sequencing.
Figures









References
-
- Caldwell RM. Rhynchosporium scald of barley, rye, and other grasses. J Agric Res. 1937;55(3):175–198.
-
- Oudemans CAJA. Observations mycologiques. Verslag Wis- en Natuurk Afd K Acad Wetensch Amsterdam. 1897;6:86–92.
-
- Frank AB. Über die Zerstörung der Gerste durch einen neuen Getreidepilz. Wochenschr Brau. 1897;42:518–520.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

