Increased Wnt5a in squamous cell lung carcinoma inhibits endothelial cell motility
- PMID: 27876017
- PMCID: PMC5120464
- DOI: 10.1186/s12885-016-2943-4
Increased Wnt5a in squamous cell lung carcinoma inhibits endothelial cell motility
Abstract
Background: Angiogenesis is important both in normal tissue function and disease and represents a key target in lung cancer (LC) therapy. Unfortunately, the two main subtypes of non-small-cell lung cancers (NSCLC) namely, adenocarcinoma (AC) and squamous cell carcinoma (SCC) respond differently to anti-angiogenic e.g. anti-vascular endothelial growth factor (VEGF)-A treatment with life-threatening side effects, often pulmonary hemorrhage in SCC. The mechanisms behind such adverse reactions are still largely unknown, although peroxisome proliferator activator receptor (PPAR) gamma as well as Wnt-s have been named as molecular regulators of the process. As the Wnt microenvironments in NSCLC subtypes are drastically different, we hypothesized that the particularly high levels of non-canonical Wnt5a in SCC might be responsible for alterations in blood vessel growth and result in serious adverse reactions.
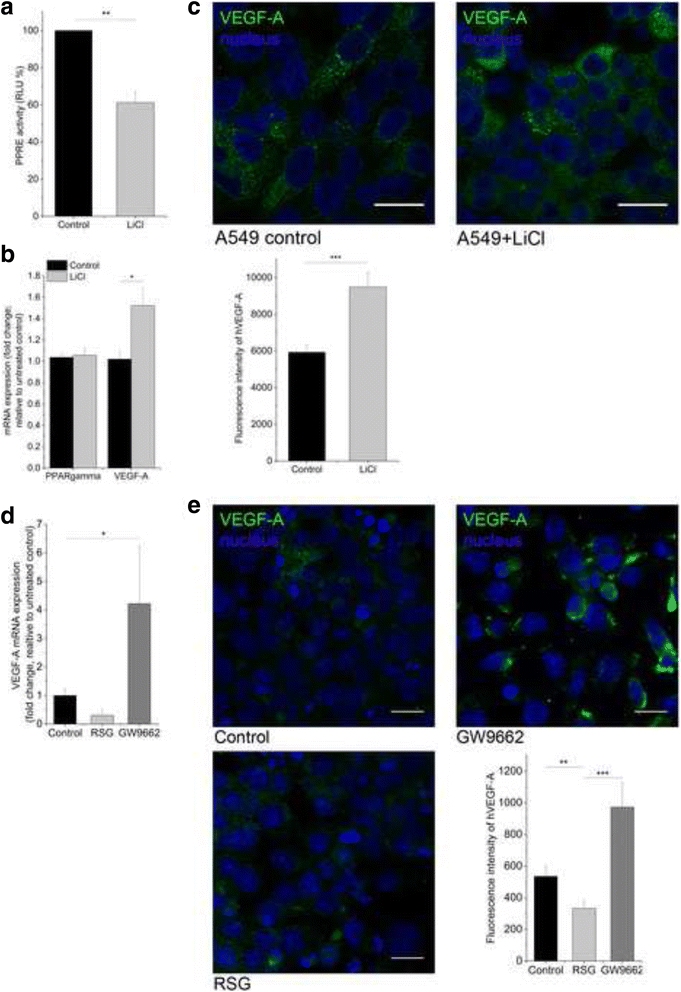
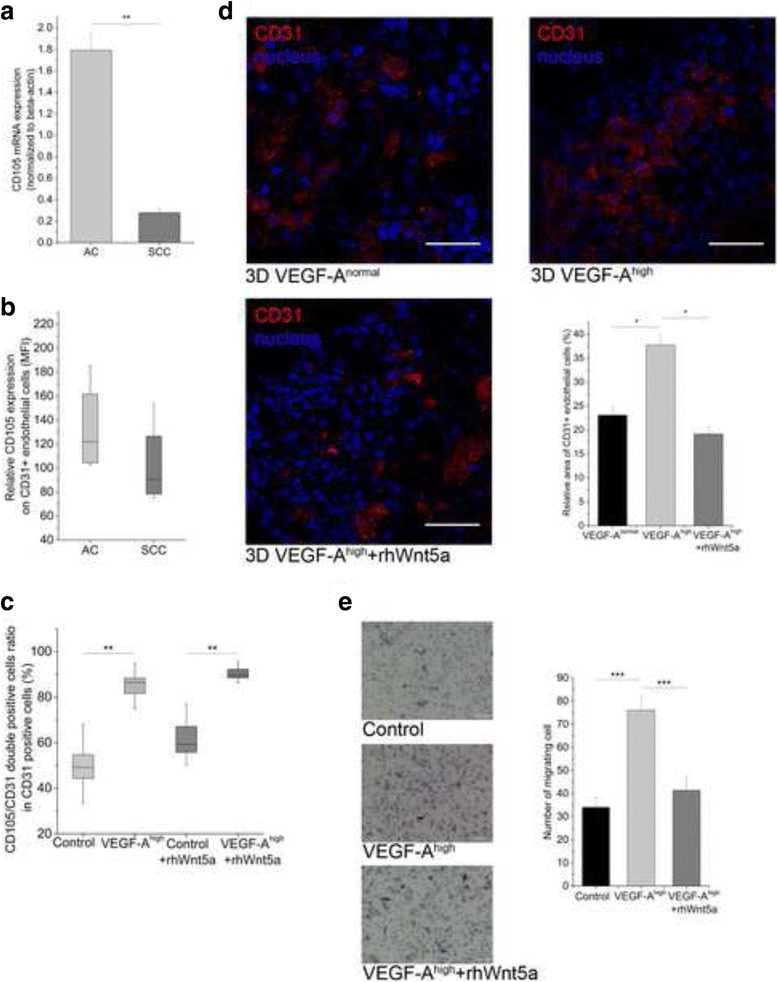
Methods: PPARgamma, VEGF-A, Wnt5a, miR-27b and miR-200b levels were determined in resected adenocarcinoma and squamous cell carcinoma samples by qRT-PCR and TaqMan microRNA assay. The role of PPARgamma in VEGF-A expression, and the role of Wnts in overall regulation was investigated using PPARgamma knock-out mice, cancer cell lines and fully human, in vitro 3 dimensional (3D), distal lung tissue aggregates. PPARgamma mRNA and protein levels were tested by qRT-PCR and immunohistochemistry, respectively. PPARgamma activity was measured by a PPRE reporter system. The tissue engineered lung tissues expressing basal level and lentivirally delivered VEGF-A were treated with recombinant Wnts, chemical Wnt pathway modifiers, and were subjected to PPARgamma agonist and antagonist treatment.
Results: PPARgamma down-regulation and VEGF-A up-regulation are characteristic to both AC and SCC. Increased VEGF-A levels are under direct control of PPARgamma. PPARgamma levels and activity, however, are under Wnt control. Imbalance of both canonical (in AC) and non-canonical (in SCC) Wnts leads to PPARgamma down-regulation. While canonical Wnts down-regulate PPARgamma directly, non-canonical Wnt5a increases miR27b that is known regulator of PPARgamma.
Conclusion: During carcinogenesis the Wnt microenvironment alters, which can downregulate PPARgamma leading to increased VEGF-A expression. Differences in the Wnt microenvironment in AC and SCC of NSCLC lead to PPARgamma decrease via mechanisms that differentially alter endothelial cell motility and branching which in turn can influence therapeutic response.
Keywords: Angiogenesis; Lung cancer; NSCLC; PPARgamma; Wnt.
Figures



References
-
- Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. [Internet] 2012;10:1236–71. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23054877. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

