Generation of monoclonal antibodies against native viral proteins using antigen-expressing mammalian cells for mouse immunization
- PMID: 27876044
- PMCID: PMC5120561
- DOI: 10.1186/s12896-016-0314-5
Generation of monoclonal antibodies against native viral proteins using antigen-expressing mammalian cells for mouse immunization
Abstract
Background: Due to their rising incidence and progressive geographical spread, infections with mosquito-borne viruses, such as dengue (DENV), chikungunya and zika virus, have developed into major public health challenges. Since all of these viruses may cause similar symptoms and can occur in concurrent epidemics, tools for their differential diagnosis and epidemiological monitoring are of urgent need.
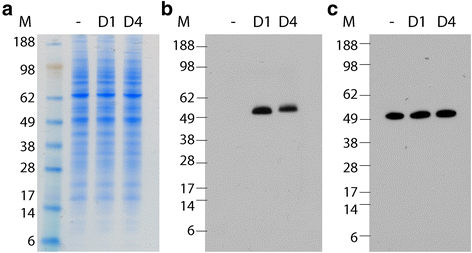
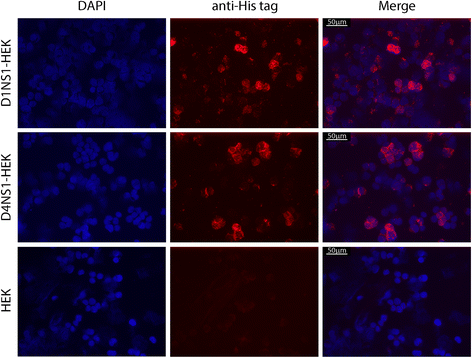
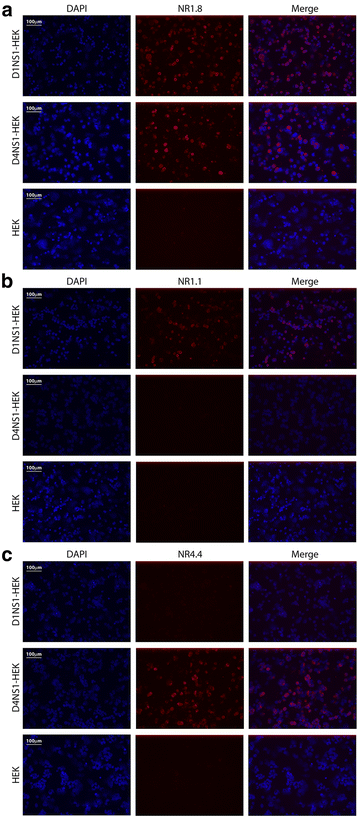
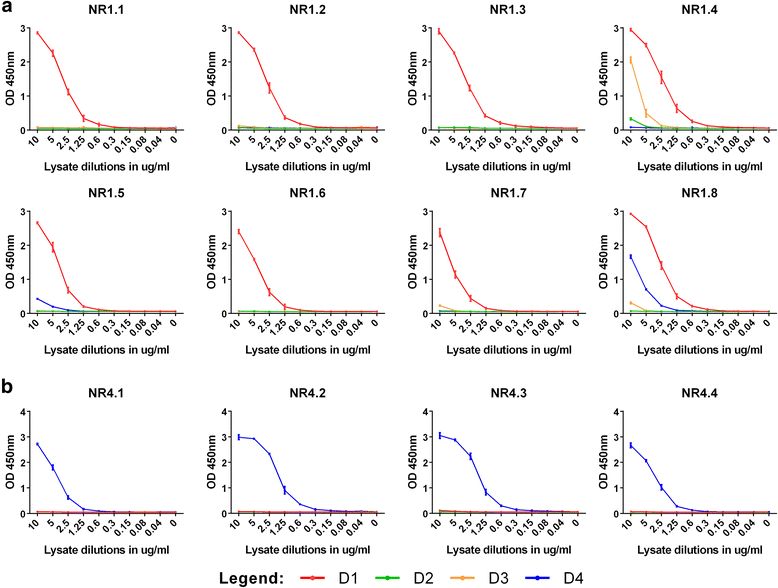
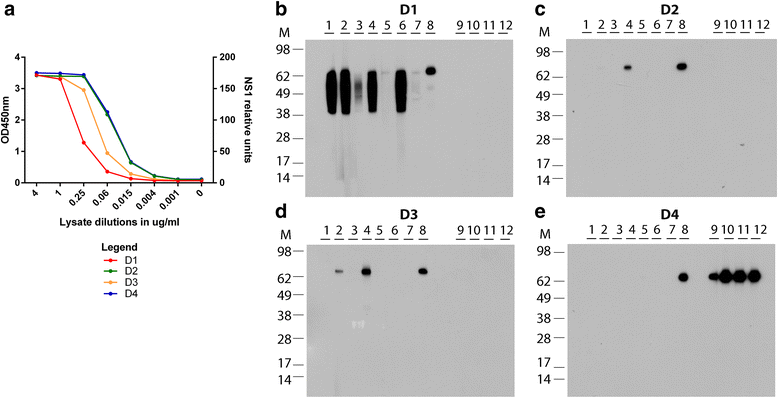
Results: Here we report the application of a novel strategy to rapidly generate monoclonal antibodies (mAbs) against native viral antigens, exemplified for the DENV nonstructural glycoprotein 1 (NS1). The described system is based on the immunization of mice with transfected mammalian cells expressing the target antigens in multiple displays on their cell surface and thereby presenting them efficiently to the host immune system in their native conformation. By applying this cell-based approach to the DENV NS1 protein of serotypes 1 (D1NS1) and 4 (D4NS1), we were able to rapidly generate panels of DENV NS1 serotype cross-reactive, as well as D1NS1- and D4NS1 serotype-specific mAbs. Our data show that the generated mAbs were capable of recognizing the endogenous NS1 protein in DENV-containing biological samples.
Conclusion: The use of this novel immunization strategy, allows for a fast and efficient generation of hybridoma cell lines, producing mAbs against native viral antigens. Envisaged applications of the mAbs include the development of test platforms enabling a differentiation of the DENV serotypes and high resolution immunotyping for epidemiological studies.
Keywords: Dengue virus; HEK cells; Hybridoma technology; Monoclonal antibodies; Mouse immunization; NS1 protein; Transfection.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

