Rotavirus vaccine effectiveness in low-income settings: An evaluation of the test-negative design
- PMID: 27876198
- PMCID: PMC5154240
- DOI: 10.1016/j.vaccine.2016.10.077
Rotavirus vaccine effectiveness in low-income settings: An evaluation of the test-negative design
Abstract
Background: The test-negative design (TND), an epidemiologic method currently used to measure rotavirus vaccine (RV) effectiveness, compares the vaccination status of rotavirus-positive cases and rotavirus-negative controls meeting a pre-defined case definition for acute gastroenteritis. Despite the use of this study design in low-income settings, the TND has not been evaluated to measure rotavirus vaccine effectiveness.
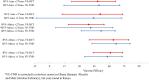
Methods: This study builds upon prior methods to evaluate the use of the TND for influenza vaccine using a randomized controlled clinical trial database. Test-negative vaccine effectiveness (VE-TND) estimates were derived from three large randomized placebo-controlled trials (RCTs) of monovalent (RV1) and pentavalent (RV5) rotavirus vaccines in sub-Saharan Africa and Asia. Derived VE-TND estimates were compared to the original RCT vaccine efficacy estimates (VE-RCTs). The core assumption of the TND (i.e., rotavirus vaccine has no effect on rotavirus-negative diarrhea) was also assessed.
Results: TND vaccine effectiveness estimates were nearly equivalent to original RCT vaccine efficacy estimates. Neither RV had a substantial effect on rotavirus-negative diarrhea.
Conclusions: This study supports the TND as an appropriate epidemiologic study design to measure rotavirus vaccine effectiveness in low-income settings.
Keywords: Rotavirus; Test-negative design; Vaccine.
Copyright © 2016 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Madhi S.A., Cunliffe N.A., Steele D., Witte D., Kirsten M., Louw C. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. - PubMed
-
- Madhi S.A., Kirsten M., Louw C., Bos P., Aspinall S., Bouckenooghe A. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine. 2012;30(Suppl. 1):A44–A51. - PubMed
-
- Armah G.E., Sow S.O., Breiman R.F., Dallas M.J., Tapia M.D., Feikin D.R. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical