Changes of bivalent chromatin coincide with increased expression of developmental genes in cancer
- PMID: 27876760
- PMCID: PMC5120258
- DOI: 10.1038/srep37393
Changes of bivalent chromatin coincide with increased expression of developmental genes in cancer
Abstract
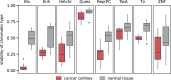
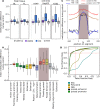
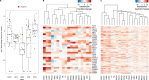
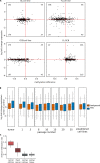
Bivalent (poised or paused) chromatin comprises activating and repressing histone modifications at the same location. This combination of epigenetic marks at promoter or enhancer regions keeps genes expressed at low levels but poised for rapid activation. Typically, DNA at bivalent promoters is only lowly methylated in normal cells, but frequently shows elevated methylation levels in cancer samples. Here, we developed a universal classifier built from chromatin data that can identify cancer samples solely from hypermethylation of bivalent chromatin. Tested on over 7,000 DNA methylation data sets from several cancer types, it reaches an AUC of 0.92. Although higher levels of DNA methylation are often associated with transcriptional silencing, counter-intuitive positive statistical dependencies between DNA methylation and expression levels have been recently reported for two cancer types. Here, we re-analyze combined expression and DNA methylation data sets, comprising over 5,000 samples, and demonstrate that the conjunction of hypermethylation of bivalent chromatin and up-regulation of the corresponding genes is a general phenomenon in cancer. This up-regulation affects many developmental genes and transcription factors, including dozens of homeobox genes and other genes implicated in cancer. Thus, we reason that the disturbance of bivalent chromatin may be intimately linked to tumorigenesis.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

