The N-terminal loop of IRAK-4 death domain regulates ordered assembly of the Myddosome signalling scaffold
- PMID: 27876844
- PMCID: PMC5120336
- DOI: 10.1038/srep37267
The N-terminal loop of IRAK-4 death domain regulates ordered assembly of the Myddosome signalling scaffold
Abstract
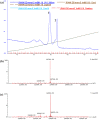
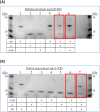
Activation of Toll-like receptors induces dimerization and the recruitment of the death domain (DD) adaptor protein MyD88 into an oligomeric post receptor complex termed the Myddosome. The Myddosome is a hub for inflammatory and oncogenic signaling and has a hierarchical arrangement with 6-8 MyD88 molecules assembling with exactly 4 of IRAK-4 and 4 of IRAK-2. Here we show that a conserved motif in IRAK-4 (Ser8-X-X-X-Arg12) is autophosphorylated and that the phosphorylated DD is unable to form Myddosomes. Furthermore a mutant DD with the phospho-mimetic residue Asp at this position is impaired in both signalling and Myddosome assembly. IRAK-4 Arg12 is also essential for Myddosome assembly and signalling and we propose that phosphorylated Ser8 induces the N-terminal loop to fold into an α-helix. This conformer is stabilised by an electrostatic interaction between phospho-Ser8 and Arg12 and would destabilise a critical interface between IRAK-4 and MyD88. Interestingly IRAK-2 does not conserve this motif and has an alternative interface in the Myddosome that requires Arg67, a residue conserved in paralogues, IRAK-1 and 3(M).
Figures





References
-
- Gay N. J., Symmons M. F., Gangloff M. & Bryant C. E. Assembly and localization of Toll-like receptor signalling complexes. Nature Rev. Immunol. 14, 546–558 (2014). - PubMed
-
- Kang J. Y. & Lee J. O. Structural biology of the Toll-like receptor family. Annu. Rev. Biochem. 80, 917–941 (2011). - PubMed
-
- Gay N. J., Gangloff M. & Weber A. N. Toll-like receptors as molecular switches. Nature Rev. Immunol. 6, 693–698 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

