Overexpression of SerpinE2/protease nexin-1 Contribute to Pathological Cardiac Fibrosis via increasing Collagen Deposition
- PMID: 27876880
- PMCID: PMC5120308
- DOI: 10.1038/srep37635
Overexpression of SerpinE2/protease nexin-1 Contribute to Pathological Cardiac Fibrosis via increasing Collagen Deposition
Abstract
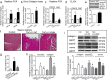
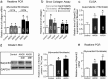
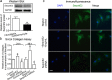
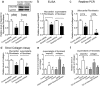
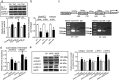
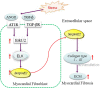
Although increases in cardiovascular load (pressure overload) are known to elicit ventricular remodeling including cardiomyocyte hypertrophy and interstitial fibrosis, the molecular mechanisms of pressure overload or AngII -induced cardiac interstitial fibrosis remain elusive. In this study, serpinE2/protease nexin-1 was over-expressed in a cardiac fibrosis model induced by pressure-overloaded via transverse aortic constriction (TAC) in mouse. Knockdown of serpinE2 attenuates cardiac fibrosis in a mouse model of TAC. At meantime, the results showed that serpinE2 significantly were increased with collagen accumulations induced by AngII or TGF-β stimulation in vitro. Intriguingly, extracellular collagen in myocardial fibroblast was reduced by knockdown of serpinE2 compared with the control in vitro. In stark contrast, the addition of exogenous PN-1 up-regulated the content of collagen in myocardial fibroblast. The MEK1/2- ERK1/2 signaling probably promoted the expression of serpinE2 via transcription factors Elk1 in myocardial fibroblast. In conclusion, stress-induced the ERK1/2 signaling pathway activation up-regulated serpinE2 expression, consequently led accumulation of collagen protein, and contributed to cardiac fibrosis.
Figures


References
-
- Bishop J. E. & Lindahl G. Regulation of cardiovascular collagen synthesis by mechanical load. Cardiovasc Res 42, 27–44 (1999). - PubMed
-
- Spinale F. G. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res 90, 520–530 (2002). - PubMed
-
- Sommer J. et al. cDNA sequence coding for a rat glia-derived nexin and its homology to members of the serpin superfamily. Biochemistry 26, 6407–6410 (1987). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

