Extended Postnatal Brain Development in the Longest-Lived Rodent: Prolonged Maintenance of Neotenous Traits in the Naked Mole-Rat Brain
- PMID: 27877105
- PMCID: PMC5099538
- DOI: 10.3389/fnins.2016.00504
Extended Postnatal Brain Development in the Longest-Lived Rodent: Prolonged Maintenance of Neotenous Traits in the Naked Mole-Rat Brain
Abstract
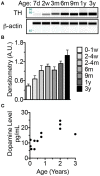
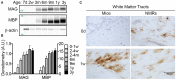
The naked mole-rat (NMR) is the longest-lived rodent with a maximum lifespan >31 years. Intriguingly, fully-grown naked mole-rats (NMRs) exhibit many traits typical of neonatal rodents. However, little is known about NMR growth and maturation, and we question whether sustained neotenous features when compared to mice, reflect an extended developmental period, commensurate with their exceptionally long life. We tracked development from birth to 3 years of age in the slowest maturing organ, the brain, by measuring mass, neural stem cell proliferation, axonal, and dendritic maturation, synaptogenesis and myelination. NMR brain maturation was compared to data from similar sized rodents, mice, and to that of long-lived mammals, humans, and non-human primates. We found that at birth, NMR brains are significantly more developed than mice, and rather are more similar to those of newborn primates, with clearly laminated hippocampi and myelinated white matter tracts. Despite this more mature brain at birth than mice, postnatal NMR brain maturation occurs at a far slower rate than mice, taking four-times longer than required for mice to fully complete brain development. At 4 months of age, NMR brains reach 90% of adult size with stable neuronal cytostructural protein expression whereas myelin protein expression does not plateau until 9 months of age in NMRs, and synaptic protein expression continues to change throughout the first 3 years of life. Intriguingly, NMR axonal composition is more similar to humans than mice whereby NMRs maintain expression of three-repeat (3R) tau even after brain growth is complete; mice experience an abrupt downregulation of 3R tau by postnatal day 8 which continues to diminish through 6 weeks of age. We have identified key ages in NMR cerebral development and suggest that the long-lived NMR may provide neurobiologists an exceptional model to study brain developmental processes that are compressed in common short-lived laboratory animal models.
Keywords: Heterocephalus glaber; comparative biology; naked mole-rat; neoteny; neurogenesis; synaptogenesis; tau.
Figures





Similar articles
-
Sustained high levels of neuroprotective, high molecular weight, phosphorylated tau in the longest-lived rodent.Neurobiol Aging. 2015 Mar;36(3):1496-504. doi: 10.1016/j.neurobiolaging.2014.12.004. Epub 2014 Dec 12. Neurobiol Aging. 2015. PMID: 25576082 Free PMC article.
-
Carcinogenesis resistance in the longest-lived rodent, the naked mole-rat.Cancer Sci. 2022 Dec;113(12):4030-4036. doi: 10.1111/cas.15570. Epub 2022 Sep 20. Cancer Sci. 2022. PMID: 36083242 Free PMC article. Review.
-
Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research.ILAR J. 2011;52(1):41-53. doi: 10.1093/ilar.52.1.41. ILAR J. 2011. PMID: 21411857
-
Protracted neuronal maturation in a long-lived, highly social rodent.PLoS One. 2022 Sep 15;17(9):e0273098. doi: 10.1371/journal.pone.0273098. eCollection 2022. PLoS One. 2022. PMID: 36107951 Free PMC article.
-
Metabolic regulatory clues from the naked mole rat: toward brain regulatory functions during stroke.Brain Res Bull. 2013 Sep;98:44-52. doi: 10.1016/j.brainresbull.2013.07.008. Epub 2013 Jul 23. Brain Res Bull. 2013. PMID: 23886571 Review.
Cited by
-
Stay social, stay young: a bioanthropological outlook on the processes linking sociality and ageing.Geroscience. 2025 Feb;47(1):721-744. doi: 10.1007/s11357-024-01416-5. Epub 2024 Nov 11. Geroscience. 2025. PMID: 39527178 Free PMC article. Review.
-
Housing and Husbandry Alternatives for Naked Mole Rat Colonies Used in Research Settings.J Am Assoc Lab Anim Sci. 2022 Sep 1;61(5):412-418. doi: 10.30802/AALAS-JAALAS-22-000035. Epub 2022 Aug 9. J Am Assoc Lab Anim Sci. 2022. PMID: 35944976 Free PMC article.
-
Insights into the Molecular Basis of Genome Stability and Pristine Proteostasis in Naked Mole-Rats.Adv Exp Med Biol. 2021;1319:287-314. doi: 10.1007/978-3-030-65943-1_11. Adv Exp Med Biol. 2021. PMID: 34424521
-
Expression of acid-sensing ion channels and selection of reference genes in mouse and naked mole rat.Mol Brain. 2016 Dec 13;9(1):97. doi: 10.1186/s13041-016-0279-2. Mol Brain. 2016. PMID: 27964758 Free PMC article.
-
Adult Neural Plasticity in Naked Mole-Rats: Implications of Fossoriality, Longevity and Sociality on the Brain's Capacity for Change.Adv Exp Med Biol. 2021;1319:105-135. doi: 10.1007/978-3-030-65943-1_4. Adv Exp Med Biol. 2021. PMID: 34424514
References
-
- Alberch P., Gould S. J., Oster G. F., Wake D. B. (1979). Size and shape in ontogeny and phylogeny. Paleobiology 5, 296–317. 10.1017/S0094837300006588 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

