Chemically engineered persistent luminescence nanoprobes for bioimaging
- PMID: 27877248
- PMCID: PMC5118608
- DOI: 10.7150/thno.16589
Chemically engineered persistent luminescence nanoprobes for bioimaging
Abstract
Imaging nanoprobes are a group of nanosized agents developed for providing improved contrast for bioimaging. Among various imaging probes, optical sensors capable of following biological events or progresses at the cellular and molecular levels are actually actively developed for early detection, accurate diagnosis, and monitoring of the treatment of diseases. The optical activities of nanoprobes can be tuned on demand by chemists by engineering their composition, size and surface nature. This review will focus on researches devoted to the conception of nanoprobes with particular optical properties, called persistent luminescence, and their use as new powerful bioimaging agents in preclinical assays.
Keywords: Nanoparticles; chemistry; persistent luminescence and in vivo imaging.; surface coating.
Conflict of interest statement
The authors have declared that no competing interest exists.
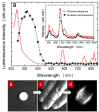
Figures
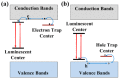
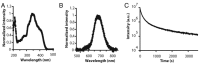
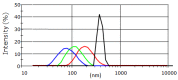

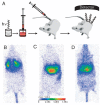
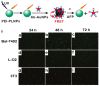
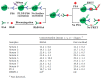


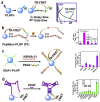
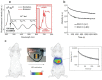


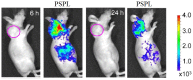
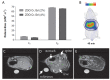
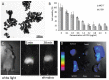
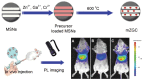
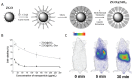
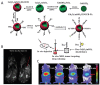
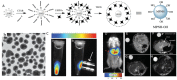
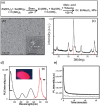
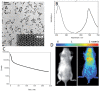

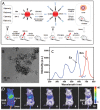

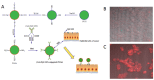
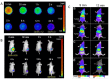
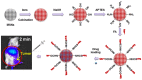

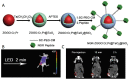
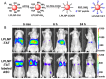
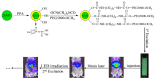

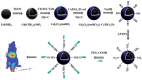
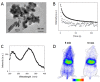
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

