A study of the synthetic methods and properties of graphenes
- PMID: 27877359
- PMCID: PMC5090618
- DOI: 10.1088/1468-6996/11/5/054502
A study of the synthetic methods and properties of graphenes
Abstract
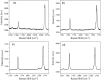
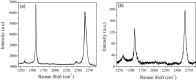
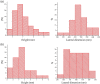
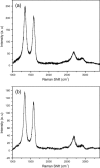
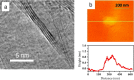
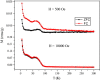
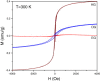
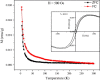
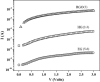
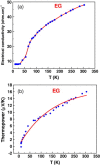
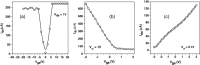
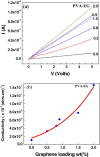
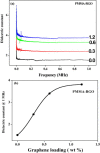
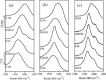
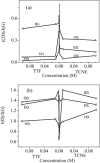
Graphenes with varying number of layers can be synthesized by using different strategies. Thus, single-layer graphene is prepared by micromechanical cleavage, reduction of single-layer graphene oxide, chemical vapor deposition and other methods. Few-layer graphenes are synthesized by conversion of nanodiamond, arc discharge of graphite and other methods. In this article, we briefly overview the various synthetic methods and the surface, magnetic and electrical properties of the produced graphenes. Few-layer graphenes exhibit ferromagnetic features along with antiferromagnetic properties, independent of the method of preparation. Aside from the data on electrical conductivity of graphenes and graphene-polymer composites, we also present the field-effect transistor characteristics of graphenes. Only single-layer reduced graphene oxide exhibits ambipolar properties. The interaction of electron donor and acceptor molecules with few-layer graphene samples is examined in detail.
Keywords: charge-transfer; electronic properties; field-effect transistor characteristics; graphenes; magnetic properties; mechanical properties; preparation methods; surface variations.
Figures






References
-
- Rao C N R, Sood A K, Voggu R. and Subrahmanyam K S. J. Phys. Chem. Lett. 2010;1:572. doi: 10.1021/jz9004174. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
