In vitro and in vivo evaluation of electrospun PCL/PMMA fibrous scaffolds for bone regeneration
- PMID: 27877567
- PMCID: PMC5090585
- DOI: 10.1088/1468-6996/14/1/015009
In vitro and in vivo evaluation of electrospun PCL/PMMA fibrous scaffolds for bone regeneration
Abstract
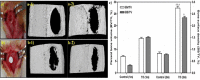
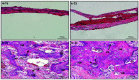
Scaffolds were fabricated by electrospinning using polycaprolactone (PCL) blended with poly(methyl methacrylate) (PMMA) in ratios of 10/0, 7/3, 5/5 and 3/7. The PCL/PMMA ratio affected the fiber diameter, contact angle, tensile strength and biological in vitro and in vivo properties of the scaffolds, and the 7/3 ratio resulted in a higher mechanical strength than 5/5 and 3/7. In vitro cytotoxicity and proliferation of MG-63 osteoblast cells on these blended scaffolds were examined by MTT assay, and it was found that PCL/PMMA blends are suitable for osteoblast cell proliferation. Confocal images and expression of proliferating cell nuclear antigen confirmed the good proliferation and expression of cells on the 7/3 PCL/PMMA fibrous scaffolds. In vivo bone formation was examined using rat models, and bone formation was observed on the 7/3 PCL/PMMA scaffold within 2 months. In vitro and in vivo results suggest that 7/3 PCL/PMMA scaffolds can be used for bone tissue regeneration.
Keywords: PCL; PMMA; bone regeneration; electrospinning.
Figures

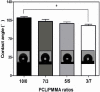
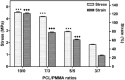
 versus stress value of 3/7 scaffold).
versus stress value of 3/7 scaffold).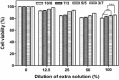






Similar articles
-
Mercaptopurine-Loaded Sandwiched Tri-Layered Composed of Electrospun Polycaprolactone/Poly(Methyl Methacrylate) Nanofibrous Scaffolds as Anticancer Carrier with Antimicrobial and Antibiotic Features: Sandwich Configuration Nanofibers, Release Study and in vitro Bioevaluation Tests.Int J Nanomedicine. 2021 Oct 11;16:6937-6955. doi: 10.2147/IJN.S332920. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34703223 Free PMC article.
-
Optimization of Polycaprolactone and Type I Collagen Scaffold for Tendon Tissue Regeneration.Cureus. 2024 Mar 25;16(3):e56930. doi: 10.7759/cureus.56930. eCollection 2024 Mar. Cureus. 2024. PMID: 38665704 Free PMC article.
-
Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering.Int J Biol Macromol. 2019 Aug 15;135:530-543. doi: 10.1016/j.ijbiomac.2019.05.204. Epub 2019 May 29. Int J Biol Macromol. 2019. PMID: 31152839
-
Electrospun polycaprolactone/gelatin composites with enhanced cell-matrix interactions as blood vessel endothelial layer scaffolds.Mater Sci Eng C Mater Biol Appl. 2017 Feb 1;71:901-908. doi: 10.1016/j.msec.2016.10.083. Epub 2016 Nov 2. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 27987787
-
A novel fibrous scaffold composed of electrospun porous poly (epsilon-caprolactone) fibers for bone tissue engineering.J Biomater Appl. 2013 Nov;28(4):514-28. doi: 10.1177/0885328212462257. Epub 2012 Oct 17. J Biomater Appl. 2013. PMID: 23075833
Cited by
-
The Role of Electrical Polarity in Electrospinning and on the Mechanical and Structural Properties of As-Spun Fibers.Materials (Basel). 2020 Sep 19;13(18):4169. doi: 10.3390/ma13184169. Materials (Basel). 2020. PMID: 32961759 Free PMC article.
-
Co-Culture of Osteoblasts and Endothelial Cells on a Microfiber Scaffold to Construct Bone-Like Tissue with Vascular Networks.Materials (Basel). 2019 Sep 5;12(18):2869. doi: 10.3390/ma12182869. Materials (Basel). 2019. PMID: 31491993 Free PMC article.
-
Multifunctional scaffolds for biomedical applications: Crafting versatile solutions with polycaprolactone enriched by graphene oxide.APL Bioeng. 2024 Mar 1;8(1):016115. doi: 10.1063/5.0184933. eCollection 2024 Mar. APL Bioeng. 2024. PMID: 38435469 Free PMC article.
-
A one-step method for generating antimicrobial nanofibre meshes via coaxial electrospinning.Mater Adv. 2024 May 20;5(13):5561-5571. doi: 10.1039/d4ma00125g. eCollection 2024 Jul 1. Mater Adv. 2024. PMID: 38957404 Free PMC article.
-
Electropsun Polycaprolactone Fibres in Bone Tissue Engineering: A Review.Mol Biotechnol. 2021 May;63(5):363-388. doi: 10.1007/s12033-021-00311-0. Epub 2021 Mar 10. Mol Biotechnol. 2021. PMID: 33689142 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
