Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications
- PMID: 27877761
- PMCID: PMC5036481
- DOI: 10.1088/1468-6996/16/2/023501
Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications
Abstract
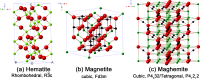
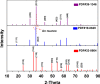
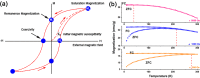
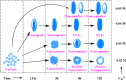
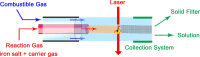
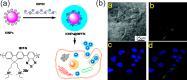
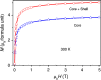
This review focuses on the recent development and various strategies in the preparation, microstructure, and magnetic properties of bare and surface functionalized iron oxide nanoparticles (IONPs); their corresponding biological application was also discussed. In order to implement the practical in vivo or in vitro applications, the IONPs must have combined properties of high magnetic saturation, stability, biocompatibility, and interactive functions at the surface. Moreover, the surface of IONPs could be modified by organic materials or inorganic materials, such as polymers, biomolecules, silica, metals, etc. The new functionalized strategies, problems and major challenges, along with the current directions for the synthesis, surface functionalization and bioapplication of IONPs, are considered. Finally, some future trends and the prospects in these research areas are also discussed.
Keywords: biomedical application; magnetic iron oxide nanoparticles; surface functional strategy.
Figures









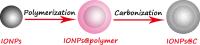







References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
