Preparation of a platinum electrocatalyst by coaxial pulse arc plasma deposition
- PMID: 27877765
- PMCID: PMC5036468
- DOI: 10.1088/1468-6996/16/2/024804
Preparation of a platinum electrocatalyst by coaxial pulse arc plasma deposition
Abstract
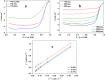
We have developed a new method of preparing Pt electrocatalysts through a dry process. By coaxial pulse arc plasma deposition (CAPD), highly ionized metal plasma can be generated from a target rod without any discharged gases, and Pt nanoparticles can be deposited on a carbon support. The small-sized Pt nanoparticles are distributed over the entire carbon surface. From transmission electron microscopy (TEM), the average size of the deposited Pt nanoparticles is estimated to be 2.5 nm, and their size distribution is narrow. Our electrocatalyst shows considerably improved catalytic activity and stability toward methanol oxidation reaction (MOR) compared with commercially available Pt catalysts such as Pt black and Pt/carbon (PtC). Inspired by its very high efficiency toward MOR, we also measured the catalytic performance for oxygen reduction reaction (ORR). Our PtC catalyst shows a better performance with half-wave potential of 0.87 V, which is higher than those of commercially available Pt catalysts. The higher performance is also supported by a right-shifted onset potential. Our preparation is simple and could be applied to other metallic nanocrystals as a novel platform in catalysis, fuel cells and biosensors.
Keywords: Pt nanoparticles; electrocatalysts; methanol oxidation.
Figures




Similar articles
-
Bimetallic Pt-Au nanocatalysts electrochemically deposited on graphene and their electrocatalytic characteristics towards oxygen reduction and methanol oxidation.Phys Chem Chem Phys. 2011 Mar 7;13(9):4083-94. doi: 10.1039/c0cp01998d. Epub 2011 Jan 13. Phys Chem Chem Phys. 2011. PMID: 21229152
-
Platinum-based oxygen reduction electrocatalysts.Acc Chem Res. 2013 Aug 20;46(8):1848-57. doi: 10.1021/ar300359w. Epub 2013 Jun 28. Acc Chem Res. 2013. PMID: 23808919 Review.
-
Pt-Pd alloy nanoparticle-decorated carbon nanotubes: a durable and methanol tolerant oxygen reduction electrocatalyst.Nanotechnology. 2012 Sep 28;23(38):385602. doi: 10.1088/0957-4484/23/38/385602. Epub 2012 Sep 4. Nanotechnology. 2012. PMID: 22948751
-
Ordered mesoporous platinum@graphitic carbon embedded nanophase as a highly active, stable, and methanol-tolerant oxygen reduction electrocatalyst.J Am Chem Soc. 2012 Feb 1;134(4):2236-45. doi: 10.1021/ja209753w. Epub 2012 Jan 17. J Am Chem Soc. 2012. PMID: 22257228
-
Mechanisms for enhanced performance of platinum-based electrocatalysts in proton exchange membrane fuel cells.ChemSusChem. 2014 Feb;7(2):361-78. doi: 10.1002/cssc.201300823. Epub 2014 Jan 21. ChemSusChem. 2014. PMID: 24449484 Review.
Cited by
-
A Review on the Promising Plasma-Assisted Preparation of Electrocatalysts.Nanomaterials (Basel). 2019 Oct 10;9(10):1436. doi: 10.3390/nano9101436. Nanomaterials (Basel). 2019. PMID: 31658708 Free PMC article. Review.
References
-
- Jung, Kim S M, Kang D H, Chung D Y, Kang Y S, Chung Y-H, Choi Y W, Pang C, Suh K-Y, Sung Y E. Chem. Mater. 2013;25:1526–32. doi: 10.1021/cm303691e. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
