Wurtzite-derived ternary I-III-O2 semiconductors
- PMID: 27877769
- PMCID: PMC5036475
- DOI: 10.1088/1468-6996/16/2/024902
Wurtzite-derived ternary I-III-O2 semiconductors
Abstract
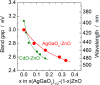
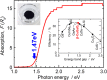
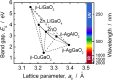
Ternary zincblende-derived I-III-VI2 chalcogenide and II-IV-V2 pnictide semiconductors have been widely studied and some have been put to practical use. In contrast to the extensive research on these semiconductors, previous studies into ternary I-III-O2 oxide semiconductors with a wurtzite-derived β-NaFeO2 structure are limited. Wurtzite-derived β-LiGaO2 and β-AgGaO2 form alloys with ZnO and the band gap of ZnO can be controlled to include the visible and ultraviolet regions. β-CuGaO2, which has a direct band gap of 1.47 eV, has been proposed for use as a light absorber in thin film solar cells. These ternary oxides may thus allow new applications for oxide semiconductors. However, information about wurtzite-derived ternary I-III-O2 semiconductors is still limited. In this paper we review previous studies on β-LiGaO2, β-AgGaO2 and β-CuGaO2 to determine guiding principles for the development of wurtzite-derived I-III-O2 semiconductors.
Keywords: 72.80.Ey; 78.40.Fy; 81.05.Dz; II–VI semiconductor; I–III–VI2 semiconductor; oxide semiconductor.
Figures

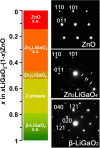
 11〉 zone axis of the hexagonal wurtzite structure for ZnO and Zn2LiGaO4 and the 〈
11〉 zone axis of the hexagonal wurtzite structure for ZnO and Zn2LiGaO4 and the 〈 01〉 zone axis of orthorhombic β-LiGaO2. The arrows in the SAED of Zn2LiGaO4 indicate superlattice diffractions.
01〉 zone axis of orthorhombic β-LiGaO2. The arrows in the SAED of Zn2LiGaO4 indicate superlattice diffractions.

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
