Perovskite-type catalytic materials for environmental applications
- PMID: 27877813
- PMCID: PMC5099850
- DOI: 10.1088/1468-6996/16/3/036002
Perovskite-type catalytic materials for environmental applications
Abstract
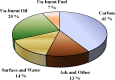
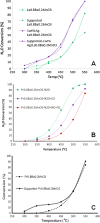
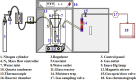
Perovskites are mixed-metal oxides that are attracting much scientific and application interest owing to their low price, adaptability, and thermal stability, which often depend on bulk and surface characteristics. These materials have been extensively explored for their catalytic, electrical, magnetic, and optical properties. They are promising candidates for the photocatalytic splitting of water and have also been extensively studied for environmental catalysis applications. Oxygen and cation non-stoichiometry can be tailored in a large number of perovskite compositions to achieve the desired catalytic activity, including multifunctional catalytic properties. Despite the extensive uses, the commercial success for this class of perovskite-based catalytic materials has not been achieved for vehicle exhaust emission control or for many other environmental applications. With recent advances in synthesis techniques, including the preparation of supported perovskites, and increasing understanding of promoted substitute perovskite-type materials, there is a growing interest in applied studies of perovskite-type catalytic materials. We have studied a number of perovskites based on Co, Mn, Ru, and Fe and their substituted compositions for their catalytic activity in terms of diesel soot oxidation, three-way catalysis, N2O decomposition, low-temperature CO oxidation, oxidation of volatile organic compounds, etc. The enhanced catalytic activity of these materials is attributed mainly to their altered redox properties, the promotional effect of co-ions, and the increased exposure of catalytically active transition metals in certain preparations. The recent lowering of sulfur content in fuel and concerns over the cost and availability of precious metals are responsible for renewed interest in perovskite-type catalysts for environmental applications.
Keywords: catalyst; diesel soot; emission control; environmental catalysis; perovskite.
Figures

References
-
- López-Suárez F E, Bueno-López A, Illán-Gómez M J. and Trawczynski J. Appl. Catal. A. 2014;485:214. doi: 10.1016/j.apcata.2014.07.037. - DOI
-
- Wua Y, Cordier C, Berrier E, Nuns N, Dujardin C. and Granger P. Appl. Catal. A. 2013;151:140. doi: 10.1016/j.apcatb.2013.04.002. - DOI
-
- Guangchuan L, Shuguang L, Changlong L. and Xiuqin O U. J. Rare Earths. 2007;25:264. doi: 10.1016/S1002-0721(07)60485-2. - DOI
-
- Basile F, Brenna G, Fornasari G, Gallob P D, Garyb D. and Vaccaria A. Stud. Surf. Sci. Catal. 2010;175:471. doi: 10.1016/S0167-2991(10)75087-4. - DOI
-
- He F, Li X, Zhao K, Huang Z, Wei G. and Li H. Fuel. 2013;108:465. doi: 10.1016/j.fuel.2012.11.035. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
