A transparent, solvent-free laminated top electrode for perovskite solar cells
- PMID: 27877878
- PMCID: PMC5102015
- DOI: 10.1080/14686996.2016.1176512
A transparent, solvent-free laminated top electrode for perovskite solar cells
Abstract
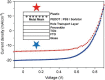
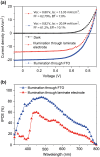
A simple lamination process of the top electrode for perovskite solar cells is demonstrated. The laminate electrode consists of a transparent and conductive plastic/metal mesh substrate, coated with an adhesive mixture of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate), PEDOT:PSS, and sorbitol. The laminate electrode showed a high degree of transparency of 85%. Best cell performance was achieved for laminate electrodes prepared with a sorbitol concentration of ~30 wt% per milliliter PEDOT:PSS dispersion, and using a pre-annealing temperature of 120°C for 10 min before lamination. Thereby, perovskite solar cells with stabilized power conversion efficiencies of (7.6 ± 1.0)% were obtained which corresponds to 80% of the reference devices with reflective opaque gold electrodes.
Keywords: 102 Porous/Nanoporous/Nanostructured materials; 209 Solar cell/Photovoltaics; 50 Energy materials; Perovskite; lamination; solar cell; transparent electrode.
Figures

References
-
- Søndergaard RR, Hösel M, Krebs FC. Roll-to-roll fabrication of large area functional organic materials. J. Polym. Sci. B: Polym. Phys. 2013;51:16. doi: 10.1002/polb.v51.1. - DOI
-
- Yang Z, Chueh C-C, Zuo F, et al. High-performance fully printable perovskite solar cells via blade-coating technique under the ambient condition. Adv. Energy Mater. 2015;5:1500328.
LinkOut - more resources
Full Text Sources
Other Literature Sources
