Lysosomal pH-inducible supramolecular dissociation of polyrotaxanes possessing acid-labile N-triphenylmethyl end groups and their therapeutic potential for Niemann-Pick type C disease
- PMID: 27877888
- PMCID: PMC5101866
- DOI: 10.1080/14686996.2016.1200948
Lysosomal pH-inducible supramolecular dissociation of polyrotaxanes possessing acid-labile N-triphenylmethyl end groups and their therapeutic potential for Niemann-Pick type C disease
Abstract
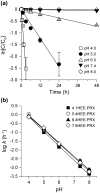
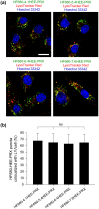
Niemann-Pick type C (NPC) disease is characterized by the accumulation of cholesterol in lysosomes. We have previously reported that biocleavable polyrotaxanes (PRXs) composed of β-cyclodextrins (β-CDs) threaded onto a linear polymer capped with bulky stopper molecules via intracellularly cleavable linkers show remarkable cholesterol reducing effects in NPC disease patient-derived fibroblasts owing to the stimuli-responsive intracellular dissociation of PRXs and subsequent β-CD release from the PRXs. Herein, we describe a series of novel acid-labile 2-(2-hydroxyethoxy)ethyl group-modified PRXs (HEE-PRXs) bearing terminal N-triphenylmethyl (N-Trt) groups as a cleavable component for the treatment of NPC disease. The N-Trt end groups of the HEE-PRXs underwent acidic pH-induced cleavage and led to the dissociation of their supramolecular structure. A kinetic study revealed that the number of HEE groups on the PRX did not affect the cleavage kinetics of the N-Trt end groups of the HEE-PRXs. The effect of the number of HEE groups of the HEE-PRXs, which was modified to impart water solubility to the PRXs, on cellular internalization efficiency, lysosomal localization efficiency, and cholesterol reduction ability in NPC disease-derived fibroblasts (NPC1 fibroblasts) was also investigated. The cellular uptake and lysosomal localization efficiency were almost equivalent for HEE-PRXs with different numbers of HEE groups. However, the cholesterol reducing ability of the HEE-PRXs in NPC1 fibroblasts was affected by the number of HEE groups, and HEE-PRXs with a high number of HEE groups were unable to reduce lysosomal cholesterol accumulation. This deficiency is most likely due to the cholesterol-solubilizing ability of HEE-modified β-CDs released from the HEE-PRXs. We conclude that the N-Trt group acts as a cleavable component to induce the lysosomal dissociation of HEE-PRXs, and acid-labile HEE-PRXs with an optimal number of HEE groups (4.1 to 5.4 HEE groups per single β-CD threaded onto the PRX) have great therapeutic potential for treating NPC disease.
Keywords: 101 Self-assembly/Self-organized materials; 211 Scaffold/Tissue engineering/Drug delivery; 30 Bio-inspired and biomedical materials; Niemann-Pick type C disease; Polyrotaxane; cholesterol; cyclodextrin; triphenylmethyl group.
Figures









References
LinkOut - more resources
Full Text Sources
Other Literature Sources
