A novel 72-kDa leukocyte-derived osteoglycin enhances the activation of toll-like receptor 4 and exacerbates cardiac inflammation during viral myocarditis
- PMID: 27878326
- PMCID: PMC5357299
- DOI: 10.1007/s00018-016-2423-7
A novel 72-kDa leukocyte-derived osteoglycin enhances the activation of toll-like receptor 4 and exacerbates cardiac inflammation during viral myocarditis
Abstract
Background: Viral myocarditis can severely damage the myocardium through excessive infiltration of immune cells. Osteoglycin (OGN) is part of the small leucine-rich repeat proteoglycan (SLRP) family. SLRP's may affect inflammatory and fibrotic processes, but the implication of OGN in cardiac inflammation and the resulting injury upon viral myocarditis is unknown.
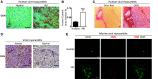
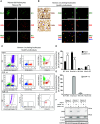
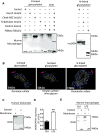
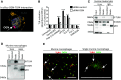
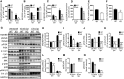
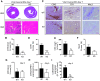
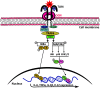
Methods and results: This study uncovered a previously unidentified 72-kDa variant of OGN that is predominant in cardiac human and mouse samples of viral myocarditis. Its absence in mice significantly decreased cardiac inflammation and injury in Coxsackievirus-B3-induced myocarditis. It also delayed mortality in lipopolysaccharide-induced endotoxemia going along with a reduced systemic production of pro-inflammatory cytokines. This 72-kDa OGN is expressed in the cell membrane of circulating and resident cardiac macrophages and neutrophils. Co-immunoprecipitation and OGN siRNA experiments revealed that this 72-kDa variant activates the toll-like receptor-4 (TLR4) with a concomitant increase in IL-6, TNF-α, IL-1β, and IL-12 expression. This immune cell activation by OGN occurred via MyD88 and increased phosphorylation of c-jun. Finally, the 72-kDa chondroitin sulfate is the result of O-linked glycosylation of the 32-kDa protein core of OGN. In contrast, the 34-kDa dermatan sulfate-OGN, involved in collagen cross linking, was also the result of O-linked glycosylation.
Conclusion: The current study discovered a novel 72-kDa chondroitin sulfate-OGN that is specific for innate immune cells. This variant is able to bind and activate TLR4. The absence of OGN decreases cytokine production by both circulating and cardiac leukocytes upon (systemic) LPS exposure, and reduces cardiac inflammation and injury in viral myocarditis.
Keywords: Glycosylation; Inflammation; Osteoglycin; TLR4; Viral myocarditis.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

