Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves
- PMID: 27878997
- PMCID: PMC5395310
- DOI: 10.1002/glia.23102
Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves
Abstract
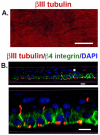
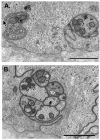
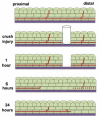
The eye is innervated by neurons derived from both the central nervous system and peripheral nervous system (PNS). While much is known about retinal neurobiology and phototransduction, less attention has been paid to the innervation of the eye by the PNS and the roles it plays in maintaining a functioning visual system. The ophthalmic branch of the trigeminal ganglion contains somas of neurons that innervate the cornea. These nerves provide sensory functions for the cornea and are referred to as intraepithelial corneal nerves (ICNs) consisting of subbasal nerves and their associated intraepithelial nerve terminals. ICNs project for several millimeters within the corneal epithelium without Schwann cell support. Here, we present evidence for the hypothesis that corneal epithelial cells function as glial cells to support the ICNs. Much of the data supporting this hypothesis is derived from studies of corneal development and the reinnervation of the ICNs in the rodent and rabbit cornea after superficial wounds. Corneal epithelial cells activate in response to injury via mechanisms similar to those induced in Schwann cells during Wallerian Degeneration. Corneal epithelial cells phagocytize distal axon fragments within hours of ICN crush wounds. During aging, the proteins, lipids, and mitochondria within the ICNs become damaged in a process exacerbated by UV light. We propose that ICNs shed their aged and damaged termini and continuously elongate to maintain their density. Available evidence points to new unexpected roles for corneal epithelial cells functioning as surrogate Schwann cells for the ICNs during homeostasis and in response to injury. GLIA 2017;65:851-863.
Keywords: Schwann cells; cornea; corneal nerves; epithelium; peripheral nervous system; wound response.
© 2016 Wiley Periodicals, Inc.
Figures






References
-
- Acosta MC, Luna C, Quirce S, Gallar J. Corneal sensory nerve activity in an experimental model of UV keratitis. Invest Ophthalmol Vis Sci. 2014;55:3403–3412. C. - PubMed
-
- Afshari FT, Kwok JC, White L, Fawcett JW. Schwann cell migration is integrin-dependent and inhibited by astrocyte-produced aggrecan. GLIA. 2010;58:857–869. - PubMed
-
- Aota S-I, Nakajima N, Sakamoto R, Watanabe S, Ibaraki N, Okazaki K. Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Dev Biol. 2003;257:1–13. - PubMed
-
- Ban D-X, Ning G-Z, Feng S-Q, Wang Y, Zhou X-H, Liu Y, Chen J-T. Combination of activated Schwann cells with bone mesenchymal stem cells: The best cell strategy for repair after spinal cord injury in rats. Regen Med. 2011;6:707–720. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

