Meiotic spindle assembly and chromosome segregation in oocytes
- PMID: 27879467
- PMCID: PMC5147004
- DOI: 10.1083/jcb.201607062
Meiotic spindle assembly and chromosome segregation in oocytes
Abstract
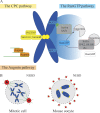
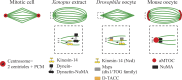
Oocytes accumulate maternal stores (proteins, mRNAs, metabolites, etc.) during their growth in the ovary to support development after fertilization. To preserve this cytoplasmic maternal inheritance, they accomplish the difficult task of partitioning their cytoplasm unequally while dividing their chromosomes equally. Added to this complexity, most oocytes, for reasons still speculative, lack the major microtubule organizing centers that most cells use to assemble and position their spindles, namely canonical centrosomes. In this review, we will address recent work on the mechanisms of meiotic spindle assembly and chromosome alignment/segregation in female gametes to try to understand the origin of errors of oocyte meiotic divisions. The challenge of oocyte divisions appears indeed not trivial because in both mice and humans oocyte meiotic divisions are prone to chromosome segregation errors, a leading cause of frequent miscarriages and congenital defects.
© 2016 Bennabi et al.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

