Chiral Hypervalent, Pentacoordinated Phosphoranes
- PMID: 27879636
- PMCID: PMC6274329
- DOI: 10.3390/molecules21111573
Chiral Hypervalent, Pentacoordinated Phosphoranes
Abstract
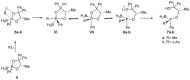
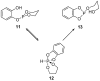
This review presents synthetic procedures applied to the preparation of chiral (mainly optically active) pentacoordinated, hypervalent mono and bicyclic phosphoranes. The mechanisms of their stereoisomerization and their selected interconversions are also presented.
Keywords: Berry pseudorotation; chirality; hypervalency; phosphoranes; turnstile rotation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
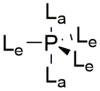


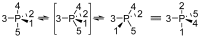





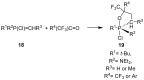











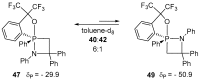
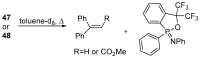




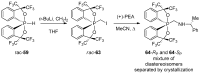





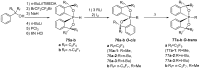
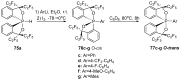
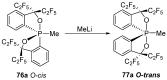



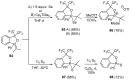





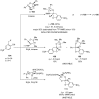





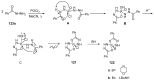
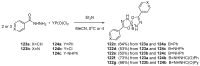


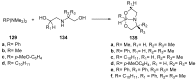
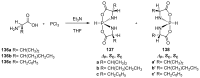

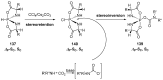


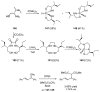

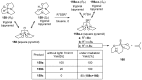







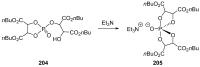
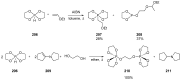
References
-
- Eliel E.L., Wilen S.H. Stereochemistry of Organic Compounds. John Wiley & Sons; New York, NY, USA: 1994.
-
- Wolf C. Dynamic Stereochemistry of Chiral Compounds: Principles and Applications. The Royal Society of Chemistry; Cambridge, UK: 2008.
-
- Singh J., Hagen T.J. Chirality and Biological Activity. In: Abraham D., Rotella D., editors. Burger’s Medicinal Chemistry and Drug Discovery. 7th ed. John Wiley & Sons; New York, NY, USA: 2010. pp. 127–166.
-
- Reddy I.K., Mehvar R. Chirality in Drug Design and Development. CRC Press; New York, NY, USA: Basel, Switzerland: 2004.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

