Evanescent field Sensors Based on Tantalum Pentoxide Waveguides - A Review
- PMID: 27879731
- PMCID: PMC3927514
- DOI: 10.3390/s8020711
Evanescent field Sensors Based on Tantalum Pentoxide Waveguides - A Review
Abstract
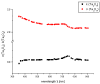
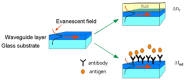
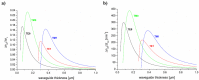
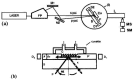
Evanescent field sensors based on waveguide surfaces play an important rolewhere high sensitivity is required. Particularly tantalum pentoxide (Ta₂O₅) is a suitablematerial for thin-film waveguides due to its high refractive index and low attenuation.Many label-free biosensor systems such as grating couplers and interferometric sensors aswell as fluorescence-based systems benefit from this waveguide material leading toextremely high sensitivity. Some biosensor systems based on Ta₂O₅ waveguides alreadytook the step into commercialization. This report reviews the various detection systems interms of limit of detection, the applications, and the suitable surface chemistry.
Keywords: Ta2O5.; biosensors; evanescent field; label-free detection.
Figures

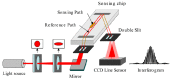

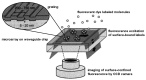

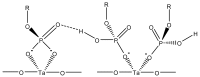
References
-
- Ehrat M., Kresbach G.M. DNA and protein microarrays and their contributions to proteomics and genomics. Chimia. 2001;55:35–39.
-
- Pawlak M., Schick E., Bopp M.A., Schneider M.J., Oroszlan P., Ehrat M. Zeptosens' protein microarrays: A novel high performance microarray platform for low abundance protein analysis. Proteomics. 2002;2:383–393. - PubMed
-
- Miklos G.L., Maleszka R. Protein functions and biological contexts. Proteomics. 2001;1:169–178. - PubMed
-
- Weissenstein U., Schneider M.J., Pawlak M., Cicenas J., Eppenberger-Castori S., Oroszlan P., Ehret S., Geurts-Moespot A., Sweep F.C.G.J., Eppenberger U. Protein chip based miniaturized assay for simultaneous quantitative monitoring of cancer biomarkers in tissue extracts. Proteomics. 2006;6:1427–1436. - PubMed
-
- Templin M.F., Stoll D., Pawlak M., Joos T.O. Protein Microarrays: Neue Systeme für die Proteomforschung. GIT Labor-Fachzeitschrift. 2006;50:890–892.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

