Imaging In Mice With Fluorescent Proteins: From Macro To Subcellular
- PMID: 27879758
- PMCID: PMC3927509
- DOI: 10.3390/s8021157
Imaging In Mice With Fluorescent Proteins: From Macro To Subcellular
Abstract
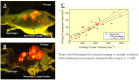
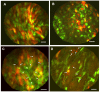
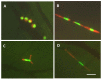
Whole-body imaging with fluorescent proteins has been shown to be a powerfultechnology with many applications in small animals. Brighter, red-shifted proteins can makewhole-body imaging even more sensitive due to reduced absorption by tissues and less scatter.For example, a new protein called Katushka has been isolated that is the brightest known proteinwith emission at wavelengths longer than 620 nm. This new protein offers potential for non-invasive whole-body macro imaging such as of tumor growth. For subcellular imaging, toobserve cytoplasmic and nuclear dynamics in the living mouse, cancer cells were labeled in thenucleus with green fluorescent protein and with red fluorescent protein in the cytoplasm. Thenuclear and cytoplasmic behavior of cancer cells in real time in blood vessels was imaged as theytrafficked by various means or adhered to the vessel surface in the abdominal skin flap. Duringextravasation, real-time dual-color imaging showed that cytoplasmic processes of the cancer cellsexited the vessels first, with nuclei following along the cytoplasmic projections. Both cytoplasmand nuclei underwent deformation during extravasation. Cancer cells trafficking in lymphaticvessels was also imaged. To noninvasively image cancer cell/stromal cell interaction in the tumormicroenvironment as well as drug response at the cellular level in live animals in real time, wedeveloped a new imageable three-color animal model. The model consists of GFP-expressingmice transplanted with the dual-color cancer cells. With the dual-color cancer cells and a highlysensitive small animal imaging system, subcellular dynamics can now be observed in live mice inreal time. Fluorescent proteins thus enable both macro and micro imaging technology and thereby provide the basis for the new field of in vivo cell biology.
Keywords: cancer cells; cellular dynamics; green fluorescent protein; in vivo cellular imaging; mice; red fluorescent protein; whole-body imaging.
Figures






References
-
- Hoffman R.M. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat. Rev. Cancer. 2005;5:796–806. - PubMed
-
- Chishima T., Miyagi Y., Wang X., Yamaoka H., Shimada H., Moossa A.R., Hoffman R.M. Cancer invasion and micrometastasis visualized in live tissue by green fluorescent protein expression. Cancer Res. 1997;57:2042–2047. - PubMed
-
- Yang M., Baranov E., Jiang P., Sun F-X., Li X-M., Li L., Hasegawa S., Bouvet M., Al-Tuwaijri M., Chishima T., Shimada H., Moossa A.R., Penman S., Hoffman R.M. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc. Natl. Acad. Sci. USA. 2000;97:1206–1211. - PMC - PubMed
-
- Yang M., Luiken G., Baranov E., Hoffman R.M. Facile whole-body imaging of internal fluorescent tumors in mice with an LED flashlight. BioTechniques. 2005;39:170–172. - PubMed
-
- Matz M.V., Fradkov A.F., Labas Y.A., Savitsky A.P., Zaraisky A.G., Markelov M.L., Lukyanov S.A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999;17:969–973. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

