Implantable Microimagers
- PMID: 27879873
- PMCID: PMC3675539
- DOI: 10.3390/s8053183
Implantable Microimagers
Abstract
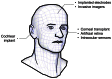
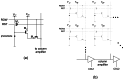
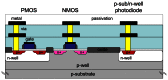
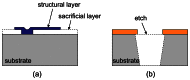
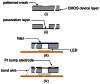
Implantable devices such as cardiac pacemakers, drug-delivery systems, and defibrillators have had a tremendous impact on the quality of live for many disabled people. To date, many devices have been developed for implantation into various parts of the human body. In this paper, we focus on devices implanted in the head. In particular, we describe the technologies necessary to create implantable microimagers. Design, fabrication, and implementation issues are discussed vis-à-vis two examples of implantable microimagers; the retinal prosthesis and in vivo neuro-microimager. Testing of these devices in animals verify the use of the microimagers in the implanted state. We believe that further advancement of these devices will lead to the development of a new method for medical and scientific applications.
Keywords: head; implant; in vivo.; microimager; retinal prosthesis.
Figures








References
-
- Greatbatch W., Holmes C.F. History of implantable devices. IEEE Eng. Med. Biol. 1991;10:38–41. 49. - PubMed
-
- Wilson B.S., Dorman M.F. Interfacing sensors with the nervous system: lessons from the development and success of the cochlear implant. IEEE Sensors J. 2008;1:131–147.
-
- Spelman F.A. Cochlear electrode arrays: past, present and future. Audiol. Neurotol. 2006;11:77–85. - PubMed
-
- Berger T.W., Baudry M., Brinton R.D., Liaw J.-S., Marmarelis V.Z., Yoondong Park A., Sheu B.J., Tanguay A.R., Jr. Brain-implantable biomimetic electronics as the next era in neuralprosthetics. P. IEEE. 2001;89:993–1012.
-
- Hochberg L.R., Serruya M.D., Friehs G.M., Mukand J.A., Saleh M., Caplan A.H., Branner A., Chen D., Penn R.D., Donoghue J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

