Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy
- PMID: 27879974
- PMCID: PMC5109842
- DOI: 10.1186/s40425-016-0177-2
Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy
Abstract
Background: Immuno-oncology (I-O) therapies target the host immune system, providing the potential to choose a uniform dose and schedule across tumor types. However, dose selection for I-O agents usually occurs early in clinical development and is typically based on tumor response, which may not fully represent the potential for improved overall survival. Here, we describe an integrated approach which incorporates clinical safety and efficacy data with data obtained from analyses of dose-/exposure-response (D-R/E-R) relationships, used to select a monotherapy dose for nivolumab, a programmed death-1 inhibitor, in clinical studies of different tumor types.
Methods: Dose was selected based on anti-tumor activity and safety data from a large phase 1b, open-label, dose-escalation study of nivolumab at doses ranging from 0.1 to 10 mg/kg administered every 2 weeks (Q2W) in 306 patients with advanced malignancies, and quantitative analyses were performed to characterize D-R/E-R relationships for pharmacodynamic, safety, and efficacy endpoints.
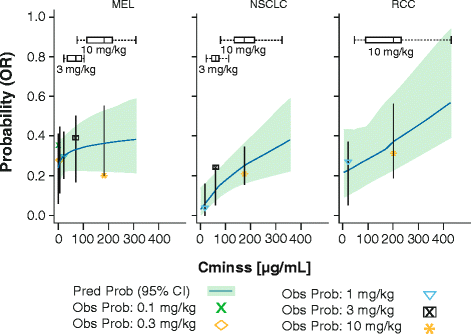
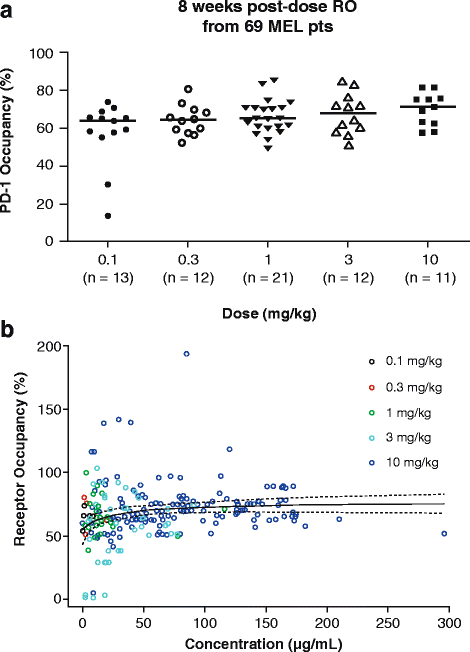
Results: A maximum tolerated dose for nivolumab was not identified, and the safety profile was similar across tumor types and dose levels (0.1-10 mg/kg). Objective response rates (ORRs) were similar across doses in melanoma and renal cell carcinoma (RCC), while higher ORRs were observed in non-small cell lung cancer (NSCLC) at 3 mg/kg and 10 mg/kg versus 1 mg/kg. Peripheral receptor occupancy was saturated at doses ≥ 0.3 mg/kg. In D-R/E-R analyses, a positive dose-dependent objective response trend was observed for each tumor type, but appeared to plateau at nivolumab doses of ≥ 1 mg/kg for melanoma and RCC, and at ≥ 3 mg/kg for NSCLC. Although there was no apparent relationship between tumor shrinkage rate and exposure, tumor progression rate appeared to decrease with increasing exposure up to a dose of 3 mg/kg Q2W for NSCLC.
Conclusions: Nivolumab monotherapy at 3 mg/kg Q2W provides unified dosing across tumor types. This dose and schedule has been validated in several phase II/III studies in which overall survival was an endpoint. Integrating D-R/E-R relationships with efficacy data and a safety profile that is unique to I-O therapy is a rational approach for dose selection of these agents.
Keywords: Dose selection; Immunotherapy; Melanoma; Nivolumab; Non-small cell lung cancer; Renal cell carcinoma.
Figures



References
-
- Hodi FS, Kluger HM, Sznol M, Carvajal RD, Lawrence DP, Atkins MB, et al. Long-term survival of ipilimumab-naïve patients with advanced melanoma treated with nivolumab in a phase 1 trial. Presented at the Society for Melanoma Research 11th Annual Meeting. Zurich; 2014.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
