Wdr68 Mediates Dorsal and Ventral Patterning Events for Craniofacial Development
- PMID: 27880803
- PMCID: PMC5120840
- DOI: 10.1371/journal.pone.0166984
Wdr68 Mediates Dorsal and Ventral Patterning Events for Craniofacial Development
Abstract
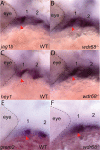
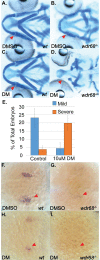
Birth defects are among the leading causes of infant mortality and contribute substantially to illness and long-term disability. Defects in Bone Morphogenetic Protein (BMP) signaling are associated with cleft lip/palate. Many craniofacial syndromes are caused by defects in signaling pathways that pattern the cranial neural crest cells (CNCCs) along the dorsal-ventral axis. For example, auriculocondylar syndrome is caused by impaired Endothelin-1 (Edn1) signaling, and Alagille syndrome is caused by defects in Jagged-Notch signaling. The BMP, Edn1, and Jag1b pathways intersect because BMP signaling is required for ventral edn1 expression that, in turn, restricts jag1b to dorsal CNCC territory. In zebrafish, the scaffolding protein Wdr68 is required for edn1 expression and subsequent formation of the ventral Meckel's cartilage as well as the dorsal Palatoquadrate. Here we report that wdr68 activity is required between the 17-somites and prim-5 stages, that edn1 functions downstream of wdr68, and that wdr68 activity restricts jag1b, hey1, and grem2 expression from ventral CNCC territory. Expression of dlx1a and dlx2a was also severely reduced in anterior dorsal and ventral 1st arch CNCC territory in wdr68 mutants. We also found that the BMP agonist isoliquiritigenin (ISL) can partially rescue lower jaw formation and edn1 expression in wdr68 mutants. However, we found no significant defects in BMP reporter induction or pSmad1/5 accumulation in wdr68 mutant cells or zebrafish. The Transforming Growth Factor Beta (TGF-β) signaling pathway is also known to be important for craniofacial development and can interfere with BMP signaling. Here we further report that TGF-β interference with BMP signaling was greater in wdr68 mutant cells relative to control cells. To determine whether interference might also act in vivo, we treated wdr68 mutant zebrafish embryos with the TGF-β signaling inhibitor SB431542 and found partial rescue of edn1 expression and craniofacial development. While ISL treatment failed, SB431542 partially rescued dlx2a expression in wdr68 mutants. Together these findings reveal an indirect role for Wdr68 in the BMP-Edn1-Jag1b signaling hierarchy and dorso-anterior expression of dlx1a/2a.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- WHO. Global strategies to reduce the health-care burden of craniofacial anomalies: World Health Organization; 2002.
-
- Rieder MJ, Green GE, Park SS, Stamper BD, Gordon CT, Johnson JM, et al. A human homeotic transformation resulting from mutations in PLCB4 and GNAI3 causes auriculocondylar syndrome. Am J Hum Genet. 2012;90(5):907–14. Epub 2012/05/09. doi: S0002-9297(12)00202-9 [pii] 10.1016/j.ajhg.2012.04.002 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

