Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis
- PMID: 27880972
- PMCID: PMC6464642
- DOI: 10.1002/14651858.CD009333.pub3
Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis
Abstract
Background: Interferons-beta (IFNs-beta) and glatiramer acetate (GA) were the first two disease-modifying therapies (DMTs) approved 20 years ago for the treatment of multiple sclerosis (MS). DMTs' prescription rates as first or switching therapies and their costs have both increased substantially over the past decade. As more DMTs become available, the choice of a specific DMT should reflect the risk/benefit profile, as well as the impact on quality of life. As MS cohorts enrolled in different studies can vary significantly, head-to-head trials are considered the best approach for gaining objective reliable data when two different drugs are compared. The purpose of this systematic review is to summarise available evidence on the comparative effectiveness of IFNs-beta and GA on disease course through the analysis of head-to-head trials.This is an update of the Cochrane review 'Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis' (first published in the Cochrane Library 2014, Issue 7).
Objectives: To assess whether IFNs-beta and GA differ in terms of safety and efficacy in the treatment of people with relapsing-remitting (RR) MS.
Search methods: We searched the Trials Register of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group (08 August 2016) and the reference lists of retrieved articles. We contacted authors and pharmaceutical companies.
Selection criteria: Randomised controlled trials (RCTs) comparing directly IFNs-beta versus GA in study participants affected by RRMS.
Data collection and analysis: We used standard methodological procedures as expected by Cochrane.
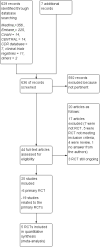
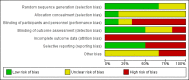
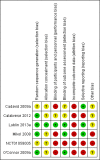
Main results: Six trials were included and five trials contributed to this review with data. A total of 2904 participants were randomly assigned to IFNs (1704) and GA (1200). The treatment duration was three years for one study, two years for the other four RCTs while one study was stopped early (after one year). The IFNs analysed in comparison with GA were IFN-beta 1b 250 mcg (two trials, 933 participants), IFN-beta 1a 44 mcg (three trials, 466 participants) and IFN-beta 1a 30 mcg (two trials, 305 participants). Enrolled participants were affected by active RRMS. All studies were at high risk for attrition bias. Three trials are still ongoing, one of them completed.Both therapies showed similar clinical efficacy at 24 months, given the primary outcome variables (number of participants with relapse (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.87 to 1.24) or progression (RR 1.11, 95% CI 0.91 to 1.35). However at 36 months, evidence from a single study suggests that relapse rates were higher in the group given IFNs than in the GA group (RR 1.40, 95% CI 1.13 to 1.74, P value 0.002).Secondary magnetic resonance imaging (MRI) outcomes analysis showed that effects on new or enlarging T2- or new contrast-enhancing T1 lesions at 24 months were similar (mean difference (MD) -0.15, 95% CI -0.68 to 0.39, and MD -0.14, 95% CI -0.30 to 0.02, respectively). However, the reduction in T2- and T1-weighted lesion volume was significantly greater in the groups given IFNs than in the GA groups (MD -0.58, 95% CI -0.99 to -0.18, P value 0.004, and MD -0.20, 95% CI -0.33 to -0.07, P value 0.003, respectively).The number of participants who dropped out of the study because of adverse events was similar in the two groups (RR 0.95, 95% CI 0.64 to 1.40).The quality of evidence for primary outcomes was judged as moderate for clinical end points, but for safety and some MRI outcomes (number of active T2 lesions), quality was judged as low.
Authors' conclusions: The effects of IFNs-beta and GA in the treatment of people with RRMS, including clinical (e.g. people with relapse, risk to progression) and MRI (Gd-enhancing lesions) measures, seem to be similar or to show only small differences. When MRI lesion load accrual is considered, the effect of the two treatments differs, in that IFNs-beta were found to limit the increase in lesion burden as compared with GA. Evidence was insufficient for a comparison of the effects of the two treatments on patient-reported outcomes, such as quality-of-life measures.
Conflict of interest statement
This Cochrane review has no commercial sponsorship.
MR and BWG have participated in meetings and trials sponsored by large pharmaceutical companies.
LLM, SF, AG, FB, GR, AL, CDP, and AV have no conflicts of interest.
Figures














Update of
-
Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis.Cochrane Database Syst Rev. 2014 Jul 26;(7):CD009333. doi: 10.1002/14651858.CD009333.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2016 Nov 24;11:CD009333. doi: 10.1002/14651858.CD009333.pub3. PMID: 25062935 Updated.
Similar articles
-
Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis.Cochrane Database Syst Rev. 2014 Jul 26;(7):CD009333. doi: 10.1002/14651858.CD009333.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2016 Nov 24;11:CD009333. doi: 10.1002/14651858.CD009333.pub3. PMID: 25062935 Updated.
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article.
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2016 Mar 22;3(3):CD009882. doi: 10.1002/14651858.CD009882.pub3. Cochrane Database Syst Rev. 2016. PMID: 27003123 Free PMC article.
-
Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2013 Jun 6;2013(6):CD008933. doi: 10.1002/14651858.CD008933.pub2. Cochrane Database Syst Rev. 2013. PMID: 23744561 Free PMC article.
-
Alemtuzumab for multiple sclerosis.Cochrane Database Syst Rev. 2016 Apr 15;4(4):CD011203. doi: 10.1002/14651858.CD011203.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2023 Jun 5;6:CD011203. doi: 10.1002/14651858.CD011203.pub3. PMID: 27082500 Free PMC article. Updated.
Cited by
-
Retrospective analysis of effectiveness of fingolimod in real life setting in Turkey (REFINE).Turk J Med Sci. 2023 Feb;53(1):323-332. doi: 10.55730/1300-0144.5588. Epub 2023 Feb 22. Turk J Med Sci. 2023. PMID: 36945929 Free PMC article.
-
Comparison of Pharmacological Therapies in Relapse Rates in Patients With Relapsing-Remitting Multiple Sclerosis.Cureus. 2023 Sep 18;15(9):e45454. doi: 10.7759/cureus.45454. eCollection 2023 Sep. Cureus. 2023. PMID: 37859931 Free PMC article. Review.
-
Interferon-β Is Less Effective Than Other Drugs in Controlling the Rate of Retinal Ganglion Cell Loss in MS.Neurol Neuroimmunol Neuroinflamm. 2021 Feb 17;8(3):e971. doi: 10.1212/NXI.0000000000000971. Print 2021 May. Neurol Neuroimmunol Neuroinflamm. 2021. PMID: 33597189 Free PMC article.
-
The Effect of Disease Modifying Therapies on Disability Progression in Multiple Sclerosis: A Systematic Overview of Meta-Analyses.Front Neurol. 2019 Jan 10;9:1150. doi: 10.3389/fneur.2018.01150. eCollection 2018. Front Neurol. 2019. PMID: 30687214 Free PMC article.
-
Interferon-β and interleukin-6 exert opposing effects on Foxp3 acetylation to control regulatory T cell induction.Front Immunol. 2025 May 30;16:1593931. doi: 10.3389/fimmu.2025.1593931. eCollection 2025. Front Immunol. 2025. PMID: 40519931 Free PMC article.
References
References to studies included in this review
Cadavid 2009a {published data only}
-
- Cadavid D, Cheriyan J, SkurnickJ, LincolnJ A, Wolansky LJ, Cook SD. New acute and chronic black holes in patients with multiple sclerosis randomised to interferon beta‐1b or glatiramer acetate. Journal Neurology Neurosurgery and Psychiatry 2009;80(12):1337‐43. - PubMed
-
- Cadavid D, Kim S, Peng B, Skurnick J, Younes M, Hill J, et al. Clinical consequences of MRI activity in treated multiple sclerosis. Multiple Sclerosis Journal 2011;17(9):1113–21. - PubMed
-
- Cadavid D, Wolansky LJ, Skurnick J, Lincoln J, Cheriyan J, Szczepanowski K, et al. Efficacy of treatment of MS with IFNbeta‐1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009;72(23):1976‐83. - PubMed
-
- Cheriyan J, Kim S, Wolansky LJ, Cook SD, Cadavid D. Impact of inflammation on brain volume in multiple sclerosis. Archives of Neurology 2012;69(1):82‐8. - PubMed
Calabrese 2012 {published data only}
-
- Calabrese M, Bernardi V, Atzori M, Mattisi I, Favaretto A, Rinaldi F, et al. Effect of disease‐modifying drugs on cortical lesions and atrophy in relapsing–remitting multiple sclerosis. Multiple Sclerosis Journal 2012;18(4):418–24. - PubMed
Lublin 2013a {published data only}
-
- Lindsey JW, Scott TF, Lynch SG, Cofield SS, Nelson F, Conwit R, et al. The CombiRx trial of combined therapy with interferon and glatiramer acetate in relapsing remitting MS: design and baseline characteristics. Multiple Sclerosis and Related Disorders 2012;1(2):81‐6. - PubMed
-
- Lublin F, Cofield S, Cutter G, Conwit R, Narayana P, Nelson F, et al. The CombiRx trial: a multi‐center, double‐blind, randomized study comparing the combined use of interferon beta‐1a and glatiramer acetate to either agent alone in participants with relapsing remitting multiple sclerosis ‐ clinical outcomes. Neurology 2012;78(1 Suppl 1):PL02.003.
-
- Lublin F, Cofield S, Cutter G, Salter A, Wang J, Conwit R, et al. EDSS changes in CombiRx: blinded, 7‐year extension results for progression and improvement. Neurology 2013;80(7 Suppl 1):P04.121.
-
- Lublin F, Cofield S, Cutter G, Salter A, Wang J, Conwit R, et al. Relapse activity in the CombiRx trial: blinded, 7‐year extension results. Neurology 2013;80(7 Suppl 1):S01.002.
Mikol 2008 {published data only}
-
- Coyle PK, Cornelisse P, Lehr L, Stubinski B. Time course of injection‐site reactions to subcutaneous interferon beta‐1a or glatiramer acetate in the REGARD Study. Proceedings of the 24th Annual Meeting of the Consortium of Multiple Sclerosis Centers, June 2–5, San Antonio, Texas, USA. 2010.
-
- Mikol DD, Barkhof F, Chang P, Coyle PK, Jeffery DR, Schwid SR, et al. Comparison of subcutaneous interferon beta‐1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open‐label trial. Lancet Neurology 2008;7(10):903‐14. - PubMed
-
- Sørensen S. REGARD: what can we learn from randomised, open‐label, head‐to‐head studies?. Lancet 2008;7(10):864‐6. - PubMed
NCT01058005 {unpublished data only}
-
- NCT01058005. Study Evaluating Rebif, Copaxone, and Tysabri for Active Multiple Sclerosis [A Multicenter, Randomized, Open‐Label, Parallel‐Group, Active‐Controlled Study to Evaluate the Benefits of Switching Therapy (Glatiramer Acetate or Interferon Beta‐1a) to Natalizumab in Subjects With Relapsing Remitting Multiple Sclerosis]. clinicaltrials.gov/show/NCT01058005 (first received 26 January 2010).
O'Connor 2009a {published data only}
-
- Goodin DS, Hartung HP, O'Connor P, Filippi M, Arnason B, Comi G, et al. Neutralizing antibodies to interferon beta‐1b multiple sclerosis: a clinico‐radiographic paradox in the beyond trial. Multiple Sclerosis 2012;18(2):181‐95. - PubMed
-
- Lampl C, Nagl S, Arnason B, Comi G, O’Connor P, Cook S, et al. Efficacy and safety of interferon beta‐1b SC in older RRMS patients‐a post hoc analysis of the BEYOND study. Journal of Neurology 2013;260(7):1838‐45. - PubMed
-
- O'Connor P, Filippi M, Arnason B, Comi G, Cook S, Goodin D, et al. 250 microg or 500 microg interferon beta‐1b versus 20 mg glatiramer acetate in relapsing‐remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurology 2009;8(10):889‐97. - PubMed
-
- O’Connor P, Arnason B, Comi GC, Filippi M, Cook S, Newark NJ, et al. Interferon beta‐1b 500 mcg, Interferon beta‐1b 250 mcg and glatiramer acetate: primary outcomes of the betaferon® efficacy yielding outcomes of a new dose study. Neurology 2008;71:LBS.004.
References to studies excluded from this review
Barbato 2011 {published data only}
-
- Barbato LM, Schofield L, McCague K, Pestreich L, Tobias K, Malhotra M, et al. Randomized, open‐label study to evaluate patient‐reported outcomes (PRO) with fingolimod after changing from prior disease‐modifying therapy (DMT) for relapsing multiple sclerosis. American Neurological Association (ANA) 136th Annual Meeting, September 25 ‐ 27, 2011; San Diego, California. 2011.
Beer 2011 {published data only}
-
- Beer K, Müller M, Hew‐Winzeler AM, Bont A, Maire P, You X, et al. The prevalence of injection‐site reactions with disease‐modifying therapies and their effect on adherence in patients with multiple sclerosis: an observational study. Bacteriologia, Virusologia, Parazitologia, Epidemiologia 2011;11(144):2‐7. - PMC - PubMed
Carra 2008 {published data only}
-
- Carra A, Onaha P, Luetic G, Burgos M, Crespo E, Deri N, et al. Therapeutic outcome 3 years after switching of immunomodulatory therapies in patients with relapsing‐remitting multiple sclerosis in Argentina. European Journal Neurology 2008;15(4):386‐93. - PubMed
Carter 2010 {published data only}
-
- Carter NJ, Keating GM. Glatiramer acetate: a review of its use in relapsing‐remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. Drugs 2010;70(12):1545‐77. - PubMed
Comi 2009 {published data only}
-
- Comi G, Martinelli V, Rodegher M, Moiola L, Bajenaru O, Carra A, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double‐blind, placebo‐controlled trial. Lancet 2009;374:1503–11. - PubMed
Comi 2011 {published data only}
-
- Comi G, Cohen JA, Arnold DL, Wynn D, Filippi M, FORTE Study Group. Phase III dose‐comparison study of glatiramer acetate for multiple sclerosis. Annals of Neurology 2011;69(1):75‐82. - PubMed
Del Santo 2011 {published data only}
-
- Santo F, Maratea D, Fadda V, Trippoli S, Messori A. Treatments for relapsing–remitting multiple sclerosis: summarising current information by network meta‐analysis. European of Journal Clinical Pharmacology 2011;68(4):441‐8. - PubMed
Ghezzi 2005 {published data only}
-
- Ghezzi A, Amato MP, Capobianco M, Gallo P, Marrosu G, Martinelli V, et al. Disease‐modifying drugs in childhood‐juvenile multiple sclerosis: results of an Italian co‐operative study. Multiple Sclerosis 2005;11(4):420‐4. - PubMed
Kalincik 2015 {published data only}
-
- Kalincik T, Jokubaitis V, Izquierdo G, Duquette P, Girard M, Grammond P, et al. Comparative effectiveness of glatiramer acetate and interferon beta formulations in relapsing‐remitting multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2015;21:1159‐71. - PubMed
Khan 2001 {published data only}
-
- Khan OA, Tselis AC, Kamholz JA, Garbern JY, Lewis RA, Lisak RP. A prospective, open‐label treatment trial to compare the effect of IFNbeta‐1a (Avonex), IFNbeta‐1b (Betaseron), and glatiramer acetate (Copaxone) on the relapse rate in relapsing‐remitting multiple sclerosis: results after 18 months of therapy. Multiple Sclerosis (Houndmills, Basingstoke, England) 2001;7(6):349‐53. - PubMed
Khan 2012 {published data only}
-
- Khan O, Bao F, Shah M, Caon C, Alexandros T, Selis A, et al. Effect of disease‐modifying therapies on brain volume in relapsing–remitting multiple sclerosis: results of a five‐year brain MRI study. Journal of the Neurological Sciences 2012;312:7–12. - PubMed
Khan 2013 {published data only}
-
- Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R, GALA Study Group. Three times weekly glatiramer acetate in relapsing‐remitting multiple sclerosis. Annals of Neurology 2013;73(6):705‐13. - PubMed
Khoury 2010 {published data only}
Ouallet 2010 {published data only}
-
- Ouallet JC. Immunomodulatory treatments for multiple sclerosis: lessons from direct comparative studies [Traitements de fond de la sclérose en plaques: enseignements des études randomisées comparatives directes]. Reveu Neurologique 2010;166(1):21‐31. - PubMed
Qizilbash 2012 {published data only}
-
- Qizilbash N, Mendez I, Sanchez‐de la Rosa R. Benefit‐risk analysis of glatiramer acetate for relapsing‐remitting and clinically isolated syndrome. Multiple Sclerosis Clinical Therapy 2012;34(1):159‐76. - PubMed
Salama 2003 {published data only}
-
- Salama HH, Abu‐Hashim EM, Bakry MA, Zhang J, Mongui A. Twelve‐month comparative study of the impacts of IFNb‐1a (Avonex), IFNb‐1b (Betaseron) and glatiramer acetate (Copaxone) on the clinical, MRI and immunological responses in relapsing‐remitting multiple sclerosis. Neurosciences (Official Journal of the Pan Arab Union of Neurological Sciences) 2003;8:93‐4.
References to ongoing studies
EUCTR2012‐003735‐32‐GR {unpublished data only}
-
- EUCTR2012‐003735‐32‐GR. Study to compare the efficacy and/or safety of masitinib to interferon beta‐1a, interferon beta‐1b, peginterferon beta‐1a or glatiramer acetate in patients with relapsing remitting multiple sclerosis with unsatisfactory response to these first line treatments [A 96‐weeks, prospective, multicentre, randomised, open label, active‐controlled, parallel groups, phase 2b/3 study to compare efficacy and safety of masitinib to first line treatment, in patients with relapsing remitting multiple sclerosis with unsatisfactory response to first line treatment]. www.clinicaltrialsregister.eu/ctr‐search/trial/2012‐003735‐32/GR. ., (first received 19 November 2015).
NCT00176592 {unpublished data only}
-
- NCT00176592. Phase IV Study, Betaseron Versus Copaxone for Relapsing Remitting or CIS Forms of MS Using Triple Dose Gad 3 T MRI [Phase IV, rater‐blinded, randomized study, comparing 250 mg of betaseron with 20 mg of copaxone in patients with the relapsing‐remitting(RR) or CIS forms of ms using 3 tesla (3T) magnetic resonance imaging (MRI) with triple‐dose gadolinium]. ClinicalTrials.gov/show/NCT00176592 (first received 13 September 2005).
NCT01623596 {published and unpublished data}
-
- NCT01623596. Evaluation of Patient Retention of Fingolimod vs. Currently Approved Disease Modifying Therapy in Patients With Relapsing Remitting Multiple Sclerosis [A 12‐month, Prospective, Randomized, Active‐controlled, Open‐label Study to Evaluate the Patient Retention of Fingolimod vs. Approved First‐line Disease Modifying Therapies in Adults With Relapsing Remitting Multiple Sclerosis (PREFERMS)]. https://clinicaltrials.gov/ct2/show/study/NCT01623596 (first received 18 June 2012).
Additional references
Alonso 2008
Cadavid 2009b
-
- Cadavid D, Cheriyan J, Skurnick J, Lincoln JA, Wolansky LJ, Cook SD. New acute and chronic black holes in patients with multiple sclerosis randomised to interferon beta‐1b or glatiramer acetate. Journal Neurology Neurosurgery and Psychiatry 2009;80(12):1337‐43. - PubMed
Cadavid 2011
-
- Cadavid D, Kim S, Peng B, Skurnick J, Younes M, Hill J, et al. Clinical consequences of MRI activity in treated multiple sclerosis. Multiple Sclerosis Journal 2011;17(9):1113–21. - PubMed
Chard 2011
-
- Chard DT, Dalton CM, Swanton J, Fisniku LK, Miszkiel KA, Thompson AJ, et al. MRI only conversion to multiple sclerosis following a clinically isolated syndrome. Journal of Neurology, Neurosurgery and Psychiatry 2011; Vol. 82, issue 2:176‐9. - PubMed
Cheriyan 2012
-
- Cheriyan J, Kim S, Wolansky LJ, Cook SD, Cadavid D. Impact of inflammation on brain volume in multiple sclerosis. Archives of Neurology 2012;69(1):82‐8. - PubMed
Comi 2001
-
- Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double‐blind, randomized, placebo‐controlled study of the effects of glatiramer acetate on magnetic resonance imaging—measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Annals of Neurology 2001;49(3):290–7. - PubMed
Compston 2008
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2008;372(9648):1502‐17. - PubMed
Coyle 2010
-
- Coyle PK, Cornelisse P, Lehr L, Stubinski B. Time course of injection‐site reactions to subcutaneous interferon beta‐1a or glatiramer acetate in the REGARD Study. Proceedings of 24th Annual Meeting of the Consortium of Multiple Sclerosis Centers, June 2–5, San Antonio, Texas, USA. 2010.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] - PubMed
Dhib‐Jalbut 2010
-
- Dhib‐Jalbut S, Marks S. Interferon beta mechanism of action in multiple sclerosis. Neurology 2010;74(1 Suppl):17‐24. - PubMed
Dubey 2016
-
- Dubey D, Cano CA, Stüve O. Update on monitoring and adverse effects of approved second‐generation disease‐modifying therapies in relapsing forms of multiple sclerosis. Current Opinion in Neurology 2016;29(3):278‐85. - PubMed
FDA 1993
-
- U.S. Food, Drug Administration. Betaseron® Product Approval Information ‐ Application No 103471‐ July 1993. http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/103471s0000_APPR... (accessed September 2016).
FDA 1996
-
- U.S. Food, Drug Administration. Avonex® Product Approval Information ‐ Application N. 103628 ‐ May 1996. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/1996/ifnbbio0517... (accessed September 2016).
FDA 2001
-
- U.S. Food, Drug Administration. Copaxone® Product Approval Information ‐ NDA 020622/S‐015 ‐ March 2001. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/020622_S015_COPAX... (accessed September 2016).
FDA 2002
-
- U.S. Food, Drug Administration. Rebif® Product Approval Information ‐ Application No 103780 ‐ March 2002. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsar... (accessed September 2016).
FDA 2009
-
- U.S. Food, Drug Administration. Extavia® Product Approval Information ‐ Application No.125290 ‐ August 2009. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/125290s0000... (accessed September 2016).
FDA 2013
-
- U.S. Food, Drug Administration. Safety ‐ What is a Serious Adverse Event?. http://www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm (accessed September 2016).
FDA 2014
-
- U.S. Food, Drug Administration. Plegridy® Product Approval Information‐ BLA 125499/S‐011‐ August 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125499s011lbl.pdf (accessed September 2016).
Filippi 2011
Filippini 2013
Fisniku 2008
-
- Fisniku LK, Chard DT, Jackson JS, Anderson VM, Altmann DR, Miszkiel KA, et al. Gray matter atrophy is related to long‐term disability in multiple sclerosis. Annals of Neurology 2008;64(3):247‐54. - PubMed
Goodin 2008a
-
- Goodin DS. Disease‐modifying therapy in multiple sclerosis. Update and clinical implications. Neurology 2008;71 Suppl 3:8–13. - PubMed
Goodin 2008b
-
- Goodin D. Comparative studies of glatiramer acetate and interferon beta. International Multiple Sclerosis Journal 2008;15(2):39‐41. - PubMed
Goodin 2012
-
- Goodin DS, Hartung HP, O'Connor P, Filippi M, Arnason B, Comi G, et al. Neutralizing antibodies to interferon beta‐1b multiple sclerosis: a clinico‐radiographic paradox in the BEYOND trial. Multiple Sclerosis (Houndmills, Basingstoke, England) 2012;18(2):181‐95. - PubMed
GRADE Working Group 2004
GRADEpro 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. GRADE Working Group, 2008.
Hadjigeorgiou 2013
-
- Hadjigeorgiou G, Doxani C, Miligkos M, Ziakas P, Bakalos G, Papadimitriou D, et al. A network meta‐analysis of randomized controlled trials for comparing the effectiveness and safety profile of treatments with marketing authorization for relapsing multiple sclerosis. Journal of Clinical Pharmacy and Therapeutics 2013;38(6):433‐9. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hillert 2012
-
- Hillert J. In the coming year we should abandon interferons and glatiramer acetate as first line therapy for MS: No. Multiple Sclerosis (Houndmills, Basingstoke, England) 2012;19(1):26‐8. - PubMed
Hutchinson 2012
-
- Hutchinson M. In the coming year we should abandon interferons and glatiramer acetate as first line therapy for MS: commentary. Multiple Sclerosis (Houndmills, Basingstoke, England) 2012;19(1):29‐30. - PubMed
IFN MSSG 1995
-
- The IFNB Multiple Sclerosis Study Group. Interferon beta‐1b is effective in relapsing‐remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double‐blind, placebo‐controlled trial. Neurology 1993;43(4):655–61. - PubMed
Jacobs 1996
-
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta‐1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Annals of Neurology 1996;39(3):285–94. - PubMed
Jelinek 2015
Johnson 1995
-
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing‐remitting multiple sclerosis: results of a phase III multi‐center, double‐blind placebo‐controlled trial: the Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45(7):1268–76. - PubMed
Kurtzke 1983
-
- Kurtzke JF. Rating neurological impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33(11):1444‐52. [MEDLINE: ] - PubMed
La Mantia 2010
Lampi 2013
-
- Lampl C, Nagl S, Arnason B, Comi G, O'Connor P, Cook S, et al. Efficacy and safety of interferon beta‐1b sc in older RRMS patients‐a posthoc analysis of the BEYOND study. Journal of Neurology 2013;260(7):1838‐45. - PubMed
Lindsey 2012
-
- Lindsey JW, Scott TF, Lynch SG, Cofield SS, Nelson F, Conwit R, et al. The CombiRx trial of combined therapy with interferon and glatiramer acetate in relapsing remitting MS: design and baseline characteristics. Multiple Sclerosis and Related Disorders 2012;1(2):81‐6. - PubMed
Lublin 2012
-
- Lublin F, Cofield S, Cutter G, Conwit R, Narayana P, Nelson F, et al. The CombiRx trial: a multi‐center, double‐blind, randomized study comparing the combined use of interferon beta‐1a and glatiramer acetate to either agent alone in participants with relapsing remitting multiple sclerosis ‐ clinical outcomes. Neurology 2012;78(1 Suppl 1):PL02.00.
Lublin 2013b
-
- Lublin F, Cofield S, Cutter G, Salter A, Wang J, Conwit R, et al. EDSS changes in CombiRx: blinded, 7‐year extension results for progression and improvement. Proceedings of the 65th American Academy of Neurology (AAN) Annual Meeting. Neurology 2013;80:1.
Lublin 2013c
-
- Lublin F, Cofield S, Cutter G, Salter A, Wang J, Conwit R, et al. Relapse activity in the CombiRx trial: blinded, 7‐year extension results. Neurology 2013;80(1 Suppl1):1.
McDonald 2001
-
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Annals of Neurology 2001;50(1):121‐7. [MEDLINE: ] - PubMed
O'Connor 2008
-
- O’Connor P, Arnason B, Comi G, Filippi M, Cook S, Goodin D, et al. Interferon beta‐1b 500 mcg, interferon beta‐1b 250 mcg and glatiramer acetate: primary outcomes of the Betaferon® efficacy yielding outcomes of a new dose study. Neurology 2008;71:153.
O'Connor 2009b
-
- O’Connor P, Filippi M, Arnason B, Comi G, Cook S, Gooding D, et al. 250 μg or 500 μg interferon beta‐1b versus 20 mg glatiramer acetate in relapsing‐remitting multiple sclerosis: a prospective, randomised, multicentre study. Errata. Lancet Neurology 2009;8:981. - PubMed
O'Connor 2011
-
- O’Connor P, Filippi M, Arnason B, Comi G, Cook S, Gooding D, et al. 250 μg or 500 μg interferon beta‐1b versus 20 mg glatiramer acetate in relapsing‐remitting multiple sclerosis: a prospective, randomised, multicentre study. Errata. Lancet Neurology 2011; Vol. 10:115. - PubMed
O'Connor 2012
-
- O’Connor P, Filippi M, Arnason B, Comi G, Cook S, Gooding D, et al. 250 μg or 500 μg interferon beta‐1b versus 20 mg glatiramer acetate in relapsing‐remitting multiple sclerosis: a prospective, randomised, multicentre study. Errata. Lancet Neurology 2012;11:27. - PubMed
Oleen‐Burkey 2013
-
- Oleen‐Burkey M, Cyhaniuk A, Swallow E. Treatment patterns in multiple sclerosis: administrative claims analysis over 10 years. Journal of Medical Economics 2013;16(3):397‐406. - PubMed
Oliver 2010
-
- Oliver BJ, Kohli E, Kasper LH. Interferon therapy in relapsing‐remitting multiple sclerosis: a systematic review and meta‐analysis of the comparative trials. Journal of the Neurological Sciences 2010;302(1‐2):96‐105. - PubMed
Parkenov 2013
-
- Parkenov V, Schluep M, Du Pasquier R. Assessing risk of multiple sclerosis therapies. Journal of Neurosurgical Sciences 2013;332(1‐2):59‐65. - PubMed
Pleimes 2013
-
- Pleimes D, Pohl C, Beckmann K, Stolz C. BEYOND Study – Data for Cochrane Analyses Protocol No. 306440. Personal communication. Bayer HealthCare Pharmaceuticals Affairs Specialized Therapeutics 2013.
Polman 2005
-
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revision to the "McDonald Criteria". Annals of Neurology 2005;58(6):840‐6. [MEDLINE: ] - PubMed
Polman 2011
Popescu 2013
-
- Popescu V, Agosta F, Hulst HE, Sluimer IC, Knol DL, Sormani MP, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry 2013;84(10):1082‐91. - PubMed
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis:guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. [MEDLINE: ] - PubMed
PRISMS 1998
-
- PRISMS Study Group. Randomised double‐blind placebo‐controlled study of interferon beta‐1a in relapsing/remitting multiple sclerosis. Lancet 1998;352:1498–504. - PubMed
Racke 2010
-
- Racke MK, Lovett‐Racke AE, Karandikar NJ. The mechanism of action of glatiramer acetate treatment in multiple sclerosis. Neurology 2010;74 Suppl 1:25‐30. - PubMed
Review Manager 2016 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rice 2001
Richards 2002
-
- Richards R, Sampson F, Beard S, Tappenden P. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technology Assessment 2002;6(18):1‐73. [MEDLINE: ] - PubMed
Rotstein 2010
-
- Rotstein DL, Mamdani M, O'Connor PW. Increasing use of disease modifying drugs for MS in Canada. Canadian Journal of the Neurological Sciences 2010;37(3):383‐8. [MEDLINE: ] - PubMed
Rudick 2009
-
- Rudick RA, Fisher E. Do interferon beta‐1b and glatiramer acetate grow brain?. Lancet Neurology 2009;8(12):1085‐6. - PubMed
Rudick 2010
-
- Rudick RA, Lee LC, Cutter GR, Miller DM, Bourdette D, Weinstock‐Guttman B, et al. Disability progression in a clinical trial of relapsing‐remitting multiple sclerosis: eight year follow‐up. Archives of Neurology 2010;67(11):1329‐35. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Signori 2016
-
- Signori A, Gallo F, Bovis F, Tullio N, Maietta I, Sormani MP. Long‐term impact of interferon or Glatiramer acetate in multiple sclerosis: A systematic review and meta‐analysis. Multiple Sclerosis and Related Disorders 2016;6:57‐63. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Sørensen 2008
-
- Sørensen S. REGARD: what can we learn from randomised, open‐label, head‐to‐head studies?. Lancet 2008; Vol. 7:864‐6. - PubMed
Vickrey 1995
-
- Vickrey BG, Hays RD, Harooni R, Harooni R, Ellison GW. A health‐related quality of life measure for multiple sclerosis. Quality of Life Research 1995;4(3):187‐206. - PubMed
Weinshenker 1989
-
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1989;112(Pt 1):133‐46. - PubMed
Weinstock‐Guttman 2008
-
- Weinstok‐Guttman B, Rmanathan M, Zwadinov R. Interferon‐beta treatment for relapsing multiple sclerosis. Expert Opinion on Biological Therapy 2008;8(9):1435‐47. [MEDLINE: ] - PubMed
Wolinsky 2012
-
- Wolinsky J, Narayana P, Nelson F, Datta S, Cofield S, Cutter G, et al. The CombiRx trial: a multi‐center, double‐blind, randomized study comparing the combined use of interferon beta‐1a and glatiramer acetate to either agent alone in participants with relapsing remitting multiple sclerosis ‐ MRI outcomes. Neurology. 2012; Vol. 78, issue 1 Suppl 1:S11.002.
Wolinsky 2013
-
- Wolinsky J, Salter A, Narayana P, Datta S, Nelson F, Cofield S, et al. MRI outcomes in CombiRx: blinded, 7‐year extension results. Neurology 2013;80(7 Suppl 1):1.
Yusuf 1985
-
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta‐blockade during and after myocardial infarction: an overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. - PubMed
Zagmutt 2013
-
- Zagmutt F, Carroll C. A network meta‐analysis assessing the rate of adverse events and drop outs of alternative treatments for relapsing forms of multiple sclerosis. Neurology. 2013; Vol. 80, issue 7 Suppl 1:1.
Zhornitsky 2015
Zivadinov 2008
-
- Zivadinov R, Reder AT, Filippi M, Minagar A, Stüve O, Lassmann H, et al. Mechanisms of action of disease‐modifying agents and brain volume changes in multiple sclerosis. Neurology 2008;71(2):136‐44. - PubMed
References to other published versions of this review
La Mantia 2014
-
- Mantia L, Pietrantonj C, Rovaris M, Rigon G, Frau S, Berardo F, Gandini A, Longobardi A, Weinstock‐Guttman B, Vaona A. Interferons‐beta versus glatiramer acetate for relapsing‐remitting multiple sclerosis. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009333.pub2] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

